St. Jude Medical, Inc. (NYSE:STJ), a global medical device company, today announced that the first patient implants occurred in the Portico™ Re-sheathable Transcatheter Aortic Valve System U.S. IDE Trial (PORTICO trial). The trial is evaluating the Portico™ Transcatheter Aortic Valve System, the first aortic heart valve that is repositionable until fully deployed. The trial will enroll patients who are considered to have a high or an extreme surgical risk (meaning they would not be considered) for open-heart surgery.
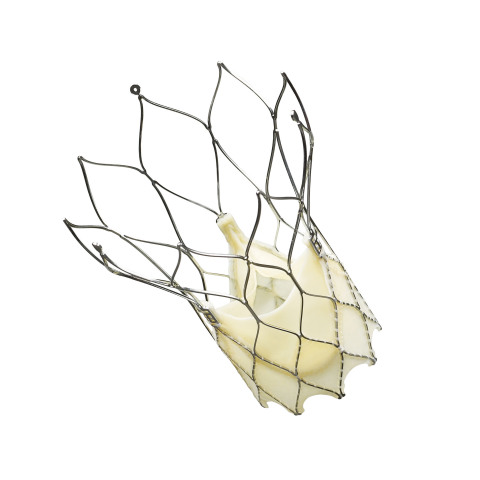
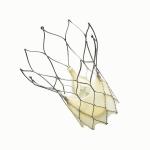
The Portico(TM) Transcatheter Aortic Heart Valve is being evaluated in a clinical study. Caution: Investigational device. Limited by Federal (or United States) law to investigational use. Photo provided by St. Jude Medical.
The PORTICO trial is evaluating the Portico valve and delivery system in patients with symptomatic severe aortic stenosis – a narrowing of the aortic valve that significantly impedes blood from flowing out of the heart. During transcatheter aortic valve replacement (TAVR) procedures, a Portico heart valve is delivered via a catheter using either a transfemoral (through the artery in the leg) or an alternative access approach in order to gain access to the heart. The Portico valve is positioned in the patient’s heart as it continues to beat, alleviating the need for cardiopulmonary bypass, where a machine takes over a patient’s heart and lung function. Patients are evaluated by a heart team consisting of a cardiac surgeon and an interventional cardiologist.
The first implants in the PORTICO trial were conducted the same day on opposite coasts of the U.S. One operating team comprised of Dr. Raj Makkar, director of Interventional Cardiology, and Dr. Wen Cheng, cardiothoracic surgeon and program director of the Thoracic Surgery Residency Program performed a Portico TAVR procedure at Cedars-Sinai Heart Institute in Los Angeles. The other operating team was comprised of Dr. Gregory P. Fontana, cardiac surgeon and chairman of the department of cardiothoracic surgery and Dr. Carlos Ruiz, an interventional cardiologist at Lenox Hill Hospital in New York City. Drs. Fontana and Makkar serve as co-principal investigators for the PORTICO trial.
“As we continue to collect clinical evidence on the best way to treat patients identified as high or at extreme risk for the open-heart valve replacement procedure, the Portico valve represents a life-saving treatment option. The valve and delivery system were designed to more safely treat heart failure symptoms in patients with stenotic valves,” said Dr. Fontana.
“The ability to fully resheath and precisely reposition the Portico valve at the implant site prior to valve deployment helps achieve accurate placement, which may simplify the implant procedure and help minimize procedural risk for the patient,” said Dr. Makkar.
St. Jude Medical incorporates more than 35 years of internally developed valve experience into the design of the Portico valve. Built on the successful Trifecta™ valve platform, the Portico valve is the first transcatheter aortic valve that can be completely resheathed (the process of bringing the valve back into the delivery catheter), repositioned at the implant site, or retrieved before being released from the delivery system.
“We have received positive feedback on the advanced features of the Portico valve from the experienced physicians who have used it. The Portico valve is an attractive option that will enable interventional cardiologists and cardiac surgeons who perform TAVR procedures to treat patients who might not otherwise be eligible for surgery,” said Dr. Mark Carlson, chief medical officer and vice president of global clinical affairs at St. Jude Medical.
Designed in collaboration with physicians, the self-expanding Portico valve is designed to maintain hemodynamics similar to that of a natural valve, while also addressing issues from early generations of transcatheter valves, such as paravalvular leak and the need for a permanent pacemaker as a result of the implant.
A prospective, multi-center, randomized, controlled clinical trial, the PORTICO trial is evaluating the safety and effectiveness of the Portico Transcatheter Aortic Valve System in reducing the risk of death and disabling stroke in patients with high or extreme surgical risk for open-heart valve replacement surgery.
Patients at up to 40 U.S. sites will be randomized based on the appropriate access method, including transfemoral, transapical (valve delivered via the left ventricle of the heart), direct aortic (through the ascending aorta), or subclavian (through an artery located below the collar bone). All trial participants will undergo a TAVR procedure receiving either a Portico valve or another commercially available TAVR valve in the U.S. Data that are collected will be used to support approval of the Portico Transcatheter Aortic Valve Replacement System by the U.S. Food and Drug Administration (FDA).
In addition to randomization, the PORTICO trial will also collect information in the form of a registry on patients with a degenerated aortic surgical bioprosthetic valve (creating a valve-in-valve registry.) These are patients in the trial who previously had valve replacement surgery and now are having a Portico valve placed inside an existing artificial valve without removing it.
The 23 mm Portico transcatheter aortic heart valve and transfemoral delivery system received a CE Mark in 2012 and the 25 mm Portico valve received a CE Mark in 2013. CE Mark clinical trials are currently underway for additional valve sizes and delivery approaches. The Portico transcatheter aortic heart valve system is limited to investigational use in the U.S., and the PORTICO trial is being conducted under an Investigational Device Exemption (IDE) from the FDA.
Aortic stenosis is the third most prevalent form of cardiovascular disease in the Western world after hypertension and coronary artery disease. Considered a potentially life-threatening condition, the aortic heart valve becomes calcified and does not open properly. Roughly 25 percent of people 65 and older, have aortic valve thickening and 3 percent age 75 and older have severe stenosis.
For additional information about the Portico valve visit SJMPortico.com and for the PORTICO trial visit clinicaltrials.gov.
About St. Jude Medical
St. Jude Medical is a global medical device manufacturer dedicated to transforming the treatment of some of the world’s most expensive, epidemic diseases. The company does this by developing cost-effective medical technologies that save and improve lives of patients around the world. Headquartered in St. Paul, Minn., St. Jude Medical has four major clinical focus areas that include cardiac rhythm management, atrial fibrillation, cardiovascular and neuromodulation. For more information, please visit sjm.com or follow us on Twitter @SJM_Media.
Forward-Looking Statements
This news release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that involve risks and uncertainties. Such forward-looking statements include the expectations, plans and prospects for the Company, including potential clinical successes, anticipated regulatory approvals and future product launches, and projected revenues, margins, earnings and market shares. The statements made by the Company are based upon management’s current expectations and are subject to certain risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. These risks and uncertainties include market conditions and other factors beyond the Company’s control and the risk factors and other cautionary statements described in the Company’s filings with the SEC, including those described in the Risk Factors and Cautionary Statements sections of the Company’s Annual Report on Form 10-K for the fiscal year ended December 28, 2013 and Quarterly Report on Form 10-Q for the fiscal quarter ended March 29, 2014. The Company does not intend to update these statements and undertakes no duty to any person to provide any such update under any circumstance.
Photos/Multimedia Gallery Available: http://www.businesswire.com/multimedia/home/20140513006725/en/
Contacts:
J.C. Weigelt, 651-756-4347
Investor
Relations
jweigelt@sjm.com
or
Micki
Sievwright, 651-756-4615
Media Relations
msievwright@sjm.com