St. Jude Medical, Inc. (NYSE:STJ), a global medical device company, and CardioMEMS, today announced U.S. Food and Drug Administration (FDA) approval of the CardioMEMS™ HF System. The CardioMEMS HF System is the first and only FDA-approved heart failure (HF) monitoring device that has been proven to significantly reduce HF hospital admissions when used by physicians to manage heart failure.
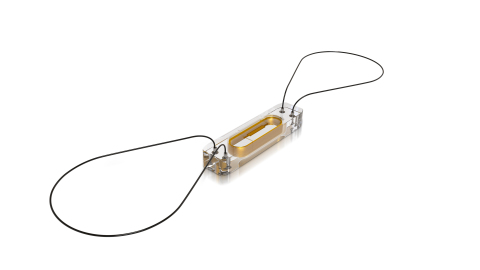
The CardioMEMS HF System uses a miniaturized, wireless monitoring sensor that is implanted in the pulmonary artery. (Photo: Business Wire)
The CardioMEMS HF System uses a miniaturized, wireless monitoring sensor that is implanted in the pulmonary artery (PA) during a minimally invasive procedure to directly measure PA pressure. Directly measuring PA pressure via a procedure called a right-heart catheterization is a standard-of-care practice for managing worsening HF in patients who have been hospitalized. The CardioMEMS HF System allows patients to transmit the same information from their homes to their health care providers allowing for personalized and proactive management to reduce the likelihood of hospitalization.
Heart failure occurs when the heart is unable to pump enough blood to meet the body’s demands and blood pressure within the heart is elevated. Significant HF progression over a period of days is known as acute decompensation and leads to hospitalization. Increased PA pressures often precede indirect measures of worsening HF such as weight and blood pressure changes. The CardioMEMS HF System allows clinicians to stabilize PA pressures by proactively managing medications and other treatment options while also providing an early indication of worsening HF.
“I believe this strategy has the potential over time to change heart failure. Not just to lighten the burdens every day or to decrease the number of hospitalizations, but to decrease the grim progression of the disease,” said Dr. Lynne Warner Stevenson, director of the Heart Failure Program at Brigham and Women’s Hospital in Boston.
Eric S. Fain, M.D., group president of St. Jude Medical, congratulates CardioMEMS on this historic accomplishment in the treatment of heart failure, and said, “The CardioMEMS HF System will not only improve the lives of patients but will also reduce the economic burden of this epidemic disease and we are delighted to have CardioMEMS become a part of St. Jude Medical.”
“We want to thank the patients and clinicians who worked with us to prove the benefit of the CardioMEMS HF System. We also want to thank FDA for making this technology available to heart failure patients in the United States,” said Jay S. Yadav, M.D., founder and CEO of CardioMEMS. “The CardioMEMS system creates a new paradigm for proactive management of heart failure and will greatly benefit patients and their families. We are excited to be working with St. Jude Medical, whose geographic reach and focus in the area of heart failure will bring this much needed treatment to patients around the world.”
The CardioMEMS HF System is supported by strong clinical evidence, including data from the CHAMPION (“CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Patients”) trial published in The Lancet. The randomized, controlled CHAMPION clinical trial proved the effectiveness of the CardioMEMS HF System in New York Heart Association (NYHA) Functional Classification System class III HF patients who had been hospitalized for HF in the previous 12 months. In contrast to large-scale studies of tele-monitoring of weight, blood pressure and transthoracic impedance such as Tele-HF, TIM-HF and DOT-HF, the CHAMPION study showed that management based on PA pressures led to a clinically significant reduction in HF admissions. Specifically, the trial demonstrated a statistically and clinically significant 28 percent reduction in the rate of HF hospitalizations at six months, and 37 percent reduction in HF hospitalizations during an average follow-up duration of 15 months.
Commenting on the CHAMPION results, Fain said, “Unlike tele-monitoring of weight and blood pressure, transthoracic impedance or intensified clinic follow-up, the CardioMEMS HF System is the only FDA-approved monitoring technology that has demonstrated the ability to significantly reduce heart failure hospitalizations in a large-scale clinical trial. This one-time implant, delivered using a catheter-based procedure, will allow physicians to proactively manage pressures to an individually tailored target rather than reacting to symptoms once a patient’s heart failure has worsened.”
The Centers for Medicare & Medicaid Services (CMS) have implemented initiatives to promote better outcomes, patient safety, and effective care by requiring hospitals to collect data on various quality metrics, most notably HF readmission rates. These data are publicly available and the purpose is to increase hospitals’ accountability for quality outcomes in order to preserve reimbursement for patient care. The CHAMPION trial demonstrates that the CardioMEMS HF System provides both clinical and economic benefits for the health care community.
About the Acquisition
In September 2010, St. Jude Medical invested $60 million for 19 percent ownership of CardioMEMS, with an exclusive option to purchase the remaining 81 percent of the company for $375 million. St. Jude Medical now intends to immediately exercise its exclusive option to acquire CardioMEMS, and expects to complete the acquisition in the second quarter of 2014.
BofA Merrill Lynch will act as financial advisor and Gibson, Dunn & Crutcher LLP as legal counsel to St. Jude Medical. J.P. Morgan Securities LLC will act as financial advisor to CardioMEMS and Cooley LLP as legal counsel.
Tweet This: #StJudeMedical and #CardioMEMS announce FDA approval of #heartfailure monitoring solution sjm.com/cardiomemsmedia via @SJM_Media
About Heart Failure
More than 5 million Americans have heart failure (HF) with 670,000 new cases diagnosed each year. Roughly 1.4 million patients in the U.S. have NYHA Class III HF, and historically these patients account for nearly half of all HF hospitalizations. According to the American Heart Association, the estimated direct and indirect cost of HF in the U.S. for 2012 was $31 billion and that number is expected to more than double by 2030.
About CardioMEMS, Inc.
Headquartered in Atlanta, CardioMEMS is a medical device company that has developed a proprietary wireless sensing and communication technology for the human body. The company's technology platform is designed to improve the management of severe chronic cardiovascular diseases such as heart failure, aneurysms, and hypertension. The sensors can be permanently implanted into the heart and blood vessels due to their small size, durability and lack of wires and batteries. CardioMEMS developed this technology based on the belief that frequent, on-demand, real-time monitoring of vital information enables proactive patient management leading to fewer hospitalizations, improved patient quality of life, and more efficient and cost-effective health care.
CardioMEMS was founded by Jay Yadav, M.D., an interventional cardiologist and entrepreneur. Lead investors in CardioMEMS include Arcapita Ventures, which is led by John Huntz, Boston Millennia Partners and Foundation Medical Partners. For more information, please visit www.cardiomems.com.
About St. Jude Medical
St. Jude Medical is a global medical device manufacturer dedicated to transforming the treatment of some of the world’s most expensive, epidemic diseases. The company does this by developing cost-effective medical technologies that save and improve lives of patients around the world. Headquartered in St. Paul, Minn., St. Jude Medical has four major clinical focus areas that include cardiac rhythm management, atrial fibrillation, cardiovascular and neuromodulation. For more information, please visit sjm.com or follow us on Twitter @SJM_Media.
Forward-Looking Statements
This news release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that involve risks and uncertainties. Such forward-looking statements include the expectations, plans and prospects for the Company, including potential clinical successes, anticipated regulatory approvals and future product launches, and projected revenues, margins, earnings and market shares. The statements made by the Company are based upon management’s current expectations and are subject to certain risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. These risks and uncertainties include market conditions and other factors beyond the Company’s control and the risk factors and other cautionary statements described in the Company’s filings with the SEC, including those described in the Risk Factors and Cautionary Statements sections of the Company’s Annual Report on Form 10-K for the fiscal year ended December 28, 2013 and Quarterly Report on Form 10-Q for the fiscal quarter ended March 29, 2014. The Company does not intend to update these statements and undertakes no duty to any person to provide any such update under any circumstance.
Photos/Multimedia Gallery Available: http://www.businesswire.com/multimedia/home/20140528006368/en/
Contacts:
J.C. Weigelt, 651-756-4347
Investor
Relations
jweigelt@sjm.com
or
Kristi
Warner, 651-756-2085
Media Relations
kwarner@sjm.com
or
CardioMEMS
Dan
Bauer, 678-651-2300
dbauer@cardiomems.com