Theralase Provides Update on 9 Months Post Treatment Cystoscopy Analysis
TORONTO, ONTARIO / ACCESSWIRE / November 8, 2018 / Theralase Technologies Inc. ("Theralase®" or the "Company") (TSXV: TLT) (OTCQB: TLTFF), a clinical stage pharmaceutical company dedicated to the research and development of light activated Photo Dynamic Compounds ("PDCs") and their associated drug formulations intended to safely and effectively destroy various cancers has provided an update on patient five, enrolled and treated in the recently completed Phase Ib Non-Muscle Invasive Bladder Cancer ("NMIBC") clinical study ("Study").
The Study's purpose was to evaluate TLD-1433, Theralase's lead PDC, for the primary endpoint of safety and tolerability, with a secondary endpoint of pharmacokinetics (movement and exit of drug within tissue) and an exploratory endpoint of efficacy.
Theralase's Anti-Cancer Treatment involves the instillation of a water-based solution of Theralase's lead anti-cancer PDC, TLD-1433 at the Therapeutic Dose (0.70 mg/cm2), via a catheter inserted through the urethra into the bladder of the patient, to allow the PDC to be preferentially absorbed by NMIBC tumours. The bladder is then drained of the solution, flushed with sterile water to remove non-absorbed solution and refilled with sterile water via a cystoscope. A fibre optic assembly, known as a Laser Emitter emits laser light to activate TLD-1433, while a proprietary Dosimetry System detects the emitted laser light, used for patient safety and efficacy. Both devices are inserted through the cystoscope, with the sole purpose of activating the absorbed PDC to destroy the NMIBC tumours.
The treatment was well tolerated by the patient, who demonstrated no tumour recurrence or presence of disease at the 90 day or 180 day clinical and cystoscopy assessment.
The patient has met Study endpoints demonstrating achievement of the primary, secondary and exploratory endpoints at 90 and 180 days and now at 270 days post treatment that marks a new achievement for the Company.
Arkady Mandel, M.D., Ph.D., D. Sc., Interim Chief Executive Officer and Chief Scientific Officer of Theralase stated, "This is an example of the enormous opportunity that awaits this young Company in the treatment of cancer. For NMIBC, a Complete Response ("CR") is defined by the FDA as the definitive endpoint for single-arm intravesical studies of patients who present with BCG-Unresponsive Carcinoma In-Situ ("CIS") disease, with or without resected papillary tumours. In the proposed Phase II NMIBC clinical study, the Company is providing two treatment procedures (therapeutic at Day 0 and maintenance at Day 180). The latest data on patient five is extremely encouraging, in that it demonstrates after only one treatment procedure, CR at 3, 6 and now 9 months post-treatment has been obtained. If the efficacy results are able to be demonstrated at 12 months post-treatment, in a larger patient population, conducted in a well-designed Phase II NMIBC clinical study, then the Theralase Anti-Cancer Technology has the potential to be the next gold standard in the treatment of NMIBC. The Theralase Anti-Cancer Technology is also multi-faceted, in that the technology is able to be adapted to the treatment of additional cancer indications, if successfully validated in independent clinical studies. Pending successful commencement of the Phase II NMIBC clinical study, the Company plans to investigate the commencement of an additional Phase Ib clinical study for a new cancer indication."
About the Phase II NMIBC Clinical Study:
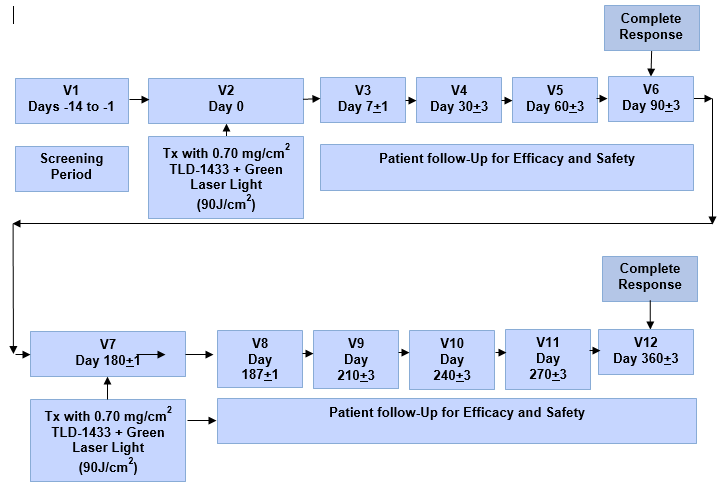
"A Phase II Clinical Study of Intravesical Photo Dynamic Therapy in Patients with BCG-Unresponsive Non-Muscle Invasive Bladder Cancer or Patients Who are Intolerant to BCG Therapy" will utilize the Therapeutic Dose (0.70 mg/cm2) of TLD-1433 and will focus on the treatment of approximately 100 NMIBC patients in approximately 20 clinical sites located in Canada, the US and internationally, with a primary endpoint of efficacy.
The endpoints of the Phase II NMIBC Clinical Study will be:
Primary (Efficacy) - Evaluated by Complete Response ("CR") in patients with Carcinoma In-Situ ("CIS") with or without resected papillary disease at 90 days post-treatment with duration of CR evaluated at 360 days post-treatment.
Patient CR is defined as at least one of the following:
1) Negative cystoscopy and negative (including atypical) urine cytology
2) Positive cystoscopy with biopsy-proven benign or low-grade NMIBC
3) Negative cystoscopy with malignant urine cytology, if cancer is found in the upper tract or prostatic urethra and random bladder biopsies are negative
Secondary (Safety) - Evaluated by the incidence and severity of Adverse Events ("AEs") Grade 4 or higher that do not resolve within 360 days post-treatment; whereby:
Grade 1 = Mild, Grade 2 = Moderate, Grade 3 = Severe, Grade 4 = Life-threatening or disabling and Grade 5 = Death
Proposed Phase II Clinical Study Treatment Plan:
About Theralase Technologies Inc.
Theralase® is a clinical stage pharmaceutical company dedicated to the research and development of light activated Photo Dynamic Compounds and their associated drug formulations intended to safely and effectively destroy various cancers.
Additional information is available at www.theralase.com and www.sedar.com.
This news release contains "forward-looking statements" which reflect the current expectations of management of the Corporation's future growth, results of operations, performance and business prospects and opportunities. Such statements include, but are not limited to, statements regarding Theralase's proposed development plans with respect to Photo Dynamic Compounds and their drug formulations. Wherever possible, words such as "may", "would", "could", "should", "will", "anticipate", "believe", "plan", "expect", "intend", "estimate", "potential for" and similar expressions have been used to identify these forward-looking statements. These statements reflect management's current beliefs with respect to future events and are based on information currently available to management. Forward-looking statements involve significant risks, uncertainties and assumptions including with respect to the ability of Theralase to: successfully fund and complete aPhase II NMIBC clinical study, secure the requisite regulatory approvals to commence and fund a Phase IINMIBC clinical study and implement its development plans. Many factors could cause the Corporation's actual results, performance or achievements to be materially different from any future results, performance or achievements that may be expressed or implied by such forward-looking statements; including, without limitation, those listed in the filings made by the Corporation with the Canadian securities regulatory authorities (which may be viewed at www.sedar.com). Should one or more of these risks or uncertainties materialize or should assumptions underlying the forward looking statements prove incorrect, actual results, performance or achievements may vary materially from those expressed or implied by the forward-looking statements contained in this news release. These factors should be considered carefully and prospective investors should not place undue reliance on the forward-looking statements. Although the forward-looking statements contained in the press release are based upon what management currently believes to be reasonable assumptions, the Corporation cannot assure prospective investors that actual results, performance or achievements will be consistent with these forward-looking statements. The Corporation disclaims any intention or obligation to revise forward-looking statements whether as a result of new information, future developments or otherwise except as required by law. All forward-looking statements are expressly qualified in their entirety by this cautionary statement.
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchanges) accepts responsibility for the adequacy or accuracy of this release.
For More Information:
1.866.THE.LASE (843-5273)
416.699.LASE (5273)
info@theralase.com
www.theralase.com
SOURCE: Theralase Technologies Inc.
View source version on accesswire.com:
https://www.accesswire.com/527458/Patient-Five-Cancer-Free-After-Single-PDT-Treatment