Results from CHAMPION-NMOSD trial demonstrated ULTOMIRIS reduced the risk of relapse in AQP4 Ab+ NMOSD by 98.6% compared to placebo
ULTOMIRIS also showed a lower proportion of patients experiencing clinically important worsening in Hauser Ambulatory Index score, a measure of patient mobility
Strong results across several subgroup analyses add to growing body of evidence supporting C5 inhibition in NMOSD
Detailed positive results from the Phase III CHAMPION-NMOSD trial showed that ULTOMIRIS® (ravulizumab-cwvz) significantly reduced relapse risk in adults with anti-aquaporin-4 (AQP4) antibody-positive (Ab+) neuromyelitis optica spectrum disorder (NMOSD), compared to the external placebo arm from the pivotal SOLIRIS® PREVENT clinical trial. Data were presented today at the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) Congress.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20221027005147/en/
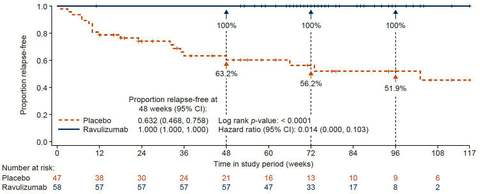
ULTOMIRIS reduced the risk of relapse by 98.6% compared with placebo (Graphic: Business Wire)
NMOSD is a rare and debilitating autoimmune disease that affects the central nervous system (CNS), including the spine and optic nerves.1-3 Most people living with NMOSD experience unpredictable relapses, characterized by a new onset of neurologic symptoms or worsening of existing neurologic symptoms, which tend to be severe and recurrent and may result in permanent disability.4-6
Sean J. Pittock, MD, Director of Mayo Clinic's Center for Multiple Sclerosis and Autoimmune Neurology and of Mayo's Neuroimmunology Laboratory and lead primary investigator in the CHAMPION-NMOSD trial, said: “The CHAMPION-NMOSD trial showed zero relapses with a median treatment duration of 73 weeks, providing evidence that ravulizumab-cwvz may offer patients sustained reduction in the risk of relapse with dosing every eight weeks and underscoring the efficacy of C5 inhibition in managing NMOSD.”
Michael Yeaman, PhD, Professor of Medicine at the UCLA School of Medicine, Director of the Institute for Infection and Immunity, Lundquist Institute at Harbor–UCLA and Chair Medical Advisor to the Guthy-Jackson Charitable Foundation for NMOSD, said: “In recent years, we have seen meaningful progress in bringing safe and effective treatments to patients with AQP4 Ab+ NMOSD, a rare disease that can disrupt many aspects of daily life, including mobility, vision, strength and balance. Based on insights from working with patients and their families every day, continued innovative research and clinical trials help empower people living with NMOSD by advancing new treatment options that may be more compatible with their individual needs and lifestyles.”
Gianluca Pirozzi, MD, PhD, Senior Vice President, Head of Development and Safety, Alexion, said: “CHAMPION-NMOSD is a remarkable example of the innovation required to design and execute rare disease clinical trials that are both scientifically rigorous and clinically meaningful. By seeking input from patients and coordinating with health authorities, this Phase III trial put the needs of patients first and evaluated measures that mattered most to them. We are excited by the significance of the results and the potential of ULTOMIRIS to advance care for the NMOSD community.”
CHAMPION-NMOSD is a global Phase III, open-label, multicenter trial evaluating the safety and efficacy of ULTOMIRIS in adults (n=58). Due to the potential long-term functional impact of NMOSD relapses and available effective treatment options, a direct placebo comparator arm was precluded for ethical reasons. ULTOMIRIS, the active treatment, was compared to the external placebo arm from the pivotal SOLIRIS PREVENT clinical trial.7
Data showed zero adjudicated relapses were observed among ULTOMIRIS patients with a median treatment duration of 73 weeks (relapse risk reduction: 98.6%, hazard ratio (95% CI): 0.014 (0.000, 0.103), p<0.0001). Additionally, 100% of patients receiving ULTOMIRIS remained relapse-free at 48 weeks, compared to 63% of patients in the external placebo arm.7
The CHAMPION-NMOSD trial also met key secondary efficacy endpoints, including adjudicated on-trial annualized relapse rate (total number of relapses in the study divided by total number of patient years) and clinically important change from baseline in mobility (ability to walk) as measured by Hauser Ambulation Index (a scale to assess mobility).7
Summary of efficacy results from primary treatment periodi,ii
Secondary Endpoint |
Statistic |
ULTOMIRIS (N=58) |
PREVENT Placebo Group (N=47) |
p Value |
Adjudicated on-trial ARR |
Adjusted ARR (95% upper CI) |
0.000 (0.044) |
N/A |
<0.0001 |
Change from baseline in HAI score |
No clinically important worsening, n (%) |
56 (96.6) |
36 (76.6) |
0.0228 |
Clinically important worsening, n (%) |
2 (3.4) |
11 (23.4) |
||
Change from baseline in EQ-5D index score |
Mean ± SD |
0.005 ± 0.1522 |
-0.043 ± 0.2115 |
0.0567 |
Median |
0.000 |
0.000 |
||
Range |
-0.33 to 0.50 |
-0.67 to 0.41 |
||
Change from baseline in EQ-5D VAS score |
Mean ± SD |
2.6 ± 14.1 |
0.6 ± 16.4 |
0.0297iii |
Median |
0.5 |
0.0 |
||
Range |
-45 to 40 |
-28 to 40 |
||
Change from baseline in EDSS score |
No clinically important worsening |
52 (89.7) |
36 (76.6) |
0.0588iii |
Clinically important worsening |
6 (10.3) |
11 (23.4) |
- Results analyzed comparing ULTOMIRIS treatment arm to the external placebo group from the PREVENT trial
- ARR, annualized relapse rate; CI, confidence limit; EDSS, Expanded Disability Status Scale; EQ-5D, EuroQol 5-Dimensions; HAI, Hauser Ambulation Index; N/A, not applicable; SD, standard deviation; VAS, visual analogue scale
- Because statistical significance for clinically important change from baseline in EQ-5D score was not met, p values for the subsequent lower-ranking secondary efficacy endpoints are not significant; therefore, nominal p values are presented.
Overall, the safety and tolerability of ULTOMIRIS was consistent with previous clinical studies and real-world use and no new safety signals were observed. The most common adverse events (AEs) (greater than or equal to 10% of patients) were COVID-19 (24%), headache (24%), back pain (12%), arthralgia (10%) and urinary tract infection (10%). All cases of COVID-19 were non-serious and considered to be unrelated to ULTOMIRIS. There were two meningococcal infections reported; both patients recovered fully with no sequelae and one continued in the trial. Fifty-six patients are continuing to receive treatment in an ongoing long-term extension period.7
CHAMPION-NMOSD subgroup and sensitivity analyses
Additional results from the CHAMPION-NMOSD trial were also presented at ECTRIMS in two posters, detailing subgroup and sensitivity analyses.
In the subgroup analysis, based on time to first adjudicated on-trial relapse, ULTOMIRIS was superior to the external placebo arm in patients receiving monotherapy (n=30; hazard ratio [HR]: 0.021; 95% confidence interval [CI]: 0.000–0.176; relapse risk reduction [RRR]: 97.9%; p<0.0001) and in patients receiving concomitant therapy (n=28; HR: 0.031; 95% CI: 0.000–0.234; RRR: 96.9%; p<0.0001). Significant differences were observed in patients who had received rituximab in the previous year (n=20; HR: 0.063; 95% CI: 0.000-0.562; RRR: 93.7%; p=0.0078) or had not (n=38; HR: 0.019; 95% CI: 0.000-0.142; RRR 98.1%; p<0.0001) compared to placebo.
The robust treatment effect of ULTOMIRIS was also observed across pre-specified efficacy subgroups, including age (<45 years or ≥45 years: RRR: 95.7–97.9%; p≤0.0012), sex (RRR: 94.3–98.2%; p≤0.0068), Asian and white races (RRR: 95.1–97.8%; p≤0.0027) and geographic region (RRR: 91.5–96.1%; p≤0.025).8
Further, pre-specified sensitivity analyses were conducted to account for potential differences in baseline patient characteristics that could impact treatment efficacy. Time to first adjudicated relapse and relapse risk reduction were analyzed using a stabilized inverse probability of treatment weighting approach. The results were consistent with the primary analysis, suggesting that any differences in baseline characteristics between the ULTOMIRIS and external placebo groups did not impact the treatment effect.9
Regulatory submissions for ULTOMIRIS for the treatment of NMOSD are currently under review with multiple health authorities, including in the United States (US), European Union (EU) and Japan.
INDICATION(S) & IMPORTANT SAFETY INFORMATION for ULTOMIRIS® (ravulizumab-cwvz)
What is ULTOMIRIS?
ULTOMIRIS is a prescription medicine used to treat:
- adults and children 1 month of age and older with a disease called Paroxysmal Nocturnal Hemoglobinuria (PNH).
- adults and children 1 month of age and older with a disease called atypical Hemolytic Uremic Syndrome (aHUS). ULTOMIRIS is not used in treating people with Shiga toxin E. coli related hemolytic uremic syndrome (STEC-HUS).
- adults with a disease called generalized myasthenia gravis (gMG) who are anti-acetylcholine receptor (AChR) antibody positive.
It is not known if ULTOMIRIS is safe and effective in children younger than 1 month of age.
It is not known if ULTOMIRIS is safe and effective for the treatment of gMG in children.
Subcutaneous administration of ULTOMIRIS has not been evaluated and is not approved for use in children.
IMPORTANT SAFETY INFORMATION
What is the most important information I should know about ULTOMIRIS?
ULTOMIRIS is a medicine that affects your immune system and can lower the ability of your immune system to fight infections.
- ULTOMIRIS increases your chance of getting serious and life-threatening meningococcal infections that may quickly become life-threatening and cause death if not recognized and treated early.
- You must receive meningococcal vaccines at least 2 weeks before your first dose of ULTOMIRIS if you are not vaccinated.
- If your doctor decided that urgent treatment with ULTOMIRIS is needed, you should receive meningococcal vaccination as soon as possible.
- If you have not been vaccinated and ULTOMIRIS therapy must be initiated immediately, you should also receive 2 weeks of antibiotics with your vaccinations.
- If you had a meningococcal vaccine in the past, you might need additional vaccination. Your doctor will decide if you need additional vaccination.
- Meningococcal vaccines reduce but do not prevent all meningococcal infections. Call your doctor or get emergency medical care right away if you get any of these signs and symptoms of a meningococcal infection: headache with nausea or vomiting, headache and fever, headache with a stiff neck or stiff back, fever, fever and a rash, confusion, muscle aches with flu-like symptoms and eyes sensitive to light.
Your doctor will give you a Patient Safety Card about the risk of meningococcal infection. Carry it with you at all times during treatment and for 8 months after your last ULTOMIRIS dose. It is important to show this card to any doctor or nurse to help them diagnose and treat you quickly.
ULTOMIRIS is only available through a program called the ULTOMIRIS REMS. Before you can receive ULTOMIRIS, your doctor must: enroll in the ULTOMIRIS REMS program; counsel you about the risk of meningococcal infection; give you information and a Patient Safety Card about the symptoms and your risk of meningococcal infection (as discussed above); and make sure that you are vaccinated with a meningococcal vaccine, and if needed, get revaccinated with the meningococcal vaccine. Ask your doctor if you are not sure if you need to be revaccinated.
ULTOMIRIS may also increase the risk of other types of serious infections. Make sure your child receives vaccinations against Streptococcus pneumoniae and Haemophilis influenzae type b (Hib) if treated with ULTOMIRIS. Call your doctor right away if you have any new signs or symptoms of infection.
Who should not receive ULTOMIRIS?
Do not receive ULTOMIRIS if you have a meningococcal infection or have not been vaccinated against meningococcal infection unless your doctor decides that urgent treatment with ULTOMIRIS is needed.
Before you receive ULTOMIRIS, tell your doctor about all of your medical conditions, including if you: have an infection or fever, are pregnant or plan to become pregnant, and are breastfeeding or plan to breastfeed. It is not known if ULTOMIRIS will harm your unborn baby or if it passes into your breast milk. You should not breastfeed during treatment and for 8 months after your final dose of ULTOMIRIS.
Tell your doctor about all the vaccines you receive and medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements which could affect your treatment.
If you have PNH and you stop receiving ULTOMIRIS, your doctor will need to monitor you closely for at least 16 weeks after you stop ULTOMIRIS. Stopping ULTOMIRIS may cause breakdown of your red blood cells due to PNH. Symptoms or problems that can happen due to red blood cell breakdown include: drop in your red blood cell count, tiredness, blood in your urine, stomach-area (abdomen) pain, shortness of breath, blood clots, trouble swallowing, and erectile dysfunction (ED) in males.
If you have aHUS, your doctor will need to monitor you closely for at least 12 months after stopping treatment for signs of worsening aHUS or problems related to a type of abnormal clotting and breakdown of your red blood cells called thrombotic microangiopathy (TMA). Symptoms or problems that can happen with TMA may include: confusion or loss of consciousness, seizures, chest pain (angina), difficulty breathing and blood clots or stroke.
ULTOMIRIS can cause serious side effects including allergic reactions to acrylic adhesive. Allergic reactions to the acrylic adhesive may happen with your subcutaneous ULTOMIRIS treatment. If you have an allergic reaction during the delivery of subcutaneous ULTOMIRIS, remove the on-body injector and get medical help right away. Your healthcare provider may treat you with medicines to help prevent or treat allergic reaction symptoms as needed.
What are the possible side effects of ULTOMIRIS?
ULTOMIRIS can cause serious side effects including infusion-related reactions. Symptoms of an infusion-related reaction with ULTOMIRIS may include lower back pain, tiredness, feeling faint, discomfort in your arms or legs, or bad taste. Tell your doctor or nurse right away if you develop these symptoms, or any other symptoms during your ULTOMIRIS infusion that may mean you are having a serious infusion reaction, including: chest pain, trouble breathing or shortness of breath, swelling of your face, tongue, or throat, and feel faint or pass out.
The most common side effects of ULTOMIRIS in people treated for PNH are upper respiratory tract infection and headache.
The most common side effects of ULTOMIRIS in people with aHUS are upper respiratory tract infection, diarrhea, nausea, vomiting, headache, high blood pressure and fever.
The most common side effects of ULTOMIRIS in people with gMG are diarrhea and upper respiratory tract infection.
The most common side effects of subcutaneous administration of ULTOMIRIS in adults treated for PNH and aHUS are local injection site reactions.
Tell your doctor about any side effect that bothers you or that does not go away. These are not all the possible side effects of ULTOMIRIS. For more information, ask your doctor or pharmacist. Call your doctor right away if you miss an ULTOMIRIS infusion or for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Read the Instructions for Use that comes with subcutaneous ULTOMIRIS for instructions about the right way to prepare and give your subcutaneous ULTOMIRIS injections through an on-body injector.
Please see the accompanying full Prescribing Information and Medication Guide for ULTOMIRIS, including Boxed WARNING regarding serious and life-threatening meningococcal infections/sepsis. Please see the accompanying Instructions for Use for the ULTOMIRIS On Body Delivery System.
Notes
NMOSD
NMOSD is a rare disease in which the immune system is inappropriately activated to target healthy tissues and cells in the CNS.1,2 Approximately three-quarters of people with NMOSD are anti-AQP4 Ab+, meaning they produce antibodies that bind to a specific protein, aquaporin-4 (AQP4).10 This binding can inappropriately activate the complement system, which is part of the immune system and is essential to the body’s defense against infection, to destroy cells in the optic nerve, spinal cord and brain.1,11,12
It most commonly affects women and begins in the mid-30s. Men and children may also develop NMOSD, but it is even more rare.13,14 People with NMOSD may experience vision problems, intense pain, loss of bladder/bowel function, abnormal skin sensations (e.g., tingling, prickling or sensitivity to heat/cold) and impact on coordination and/or movement.3-5,15,16 Most people living with NMOSD experience unpredictable relapses, also known as attacks. Each relapse can result in cumulative disability including vision loss, paralysis and sometimes premature death.4-6 NMOSD is a distinct disease from other CNS diseases, including multiple sclerosis. The journey to diagnosis can be long, with the disease sometimes misdiagnosed.17-19
CHAMPION-NMOSD
CHAMPION-NMOSD is a global Phase III, open-label, multicenter trial evaluating the safety and efficacy of ULTOMIRIS in adults with NMOSD. The trial enrolled 58 patients across North America, Europe, Asia-Pacific and Japan. Participants were required to have a confirmed NMOSD diagnosis with a positive anti-AQP4 antibody test, at least one attack or relapse in the 12 months prior to the screening visit, an Expanded Disability Status Scale Score of 7 or less and body weight of at least 40 kilograms at trial entry. Participants could stay on stable supportive immunosuppressive therapy for the duration of the trial.20
Due to the potential long-term functional impact of NMOSD relapses and available effective treatment options, a direct placebo comparator arm was precluded for ethical reasons. The active treatment was compared to an external placebo arm from the pivotal SOLIRIS PREVENT clinical trial.
Over a median treatment duration of 73 weeks, all enrolled patients received a single weight-based loading dose of ULTOMIRIS on Day 1, followed by regular weight-based maintenance dosing beginning on Day 15, every eight weeks. The primary endpoint was time to first on-trial relapse, as confirmed by an independent adjudication committee. The end of the primary treatment period could have occurred either when all patients completed or discontinued prior to the Week 26 visit and two or more adjudicated relapses were observed, or when all patients completed or discontinued prior to the Week 50 visit if fewer than two adjudicated relapses were observed. In the trial, there were zero adjudicated relapses so the end of the primary treatment period occurred when the last enrolled participant completed the 50 week visit.
Patients who completed the primary treatment period were eligible to continue into a long-term extension period, which is ongoing.
ULTOMIRIS
ULTOMIRIS (ravulizumab-cwvz), the first and only long-acting C5 complement inhibitor, provides immediate, complete and sustained complement inhibition. The medication works by inhibiting the C5 protein in the terminal complement cascade, a part of the body’s immune system. When activated in an uncontrolled manner, the complement cascade over-responds, leading the body to attack its own healthy cells. ULTOMIRIS is administered intravenously every eight weeks in adult patients, following a loading dose.
ULTOMIRIS is approved in the US, EU and Japan for the treatment of certain adults with gMG.
ULTOMIRIS is also approved in the US, EU and Japan for the treatment of certain adults with PNH and for certain children with PNH in the US and EU.
Additionally, ULTOMIRIS is approved in the US, EU and Japan for certain adults and children with aHUS to inhibit complement-mediated thrombotic microangiopathy.
As part of a broad development program, ULTOMIRIS is being assessed for the treatment of additional hematology and neurology indications.
Alexion
Alexion, AstraZeneca Rare Disease, is the group within AstraZeneca focused on rare diseases, created following the 2021 acquisition of Alexion Pharmaceuticals, Inc. As a leader in rare diseases for 30 years, Alexion is focused on serving patients and families affected by rare diseases and devastating conditions through the discovery, development and commercialization of life-changing medicines. Alexion focuses its research efforts on novel molecules and targets in the complement cascade and its development efforts on hematology, nephrology, neurology, metabolic disorders, cardiology and ophthalmology. Headquartered in Boston, Massachusetts, Alexion has offices around the globe and serves patients in more than 50 countries. For more information, please visit www.alexion.com.
About AstraZeneca
AstraZeneca is a global, science-led biopharmaceutical company that focuses on the discovery, development and commercialization of prescription medicines in Oncology, Rare Diseases and BioPharmaceuticals, including Cardiovascular, Renal & Metabolism, and Respiratory & Immunology. Based in Cambridge, UK, AstraZeneca operates in over 100 countries, and its innovative medicines are used by millions of patients worldwide. For more information, please visit www.astrazeneca-us.com and follow us on Twitter @AstraZenecaUS.
References
- Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6(9):805-815.
- Wingerchuk DM. Diagnosis and treatment of neuromyelitis optica. Neurologist. 2007;13(1):2-11.
- Hamid SHM, Whittam D, Mutch K et al. What proportion of AQP4-IgG-negative NMO spectrum disorder patients are MOG-IgG positive? A cross sectional study of 132 patients. J Neurol. 2017;264(10):2088-2094.
- Wingerchuk DM, Weinshenker BG. Neuromyelitis optica. Curr Treat Options Neurol. 2008;10(1):55-66.
- Kitley J, Leite MI, Nakashima I, et al. Prognostic factors and disease course in aquaporin-4 antibody-positive patients with neuromyelitis optica spectrum disorder from the United Kingdom and Japan. Brain. 2012;135(6):1834-1849.
- Jarius S, Ruprecht K, Wildemann B, et al. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: a multicentre study of 175 patients. J Neuroinflamm. 2012;9:14.
- Pittock SJ, Barnett M et al. Efficacy and safety of ravulizumab in adults with anti-aquaporin-4 antibody-positive neuromyelitis optica spectrum disorder: outcomes from the phase 3 CHAMPION-NMOSD trial. Oral Presentation at: European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) Congress, October 27, 2022; Presentation O051
- Pittock SJ, Barnett M et al. Efficacy subgroup analyses from the phase 3 CHAMPION-NMOSD trial in adults with anti-aquaporin-4 antibody-positive neuromyelitis optica spectrum disorder. Poster Presentation at: European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) Congress, October 26, 2022; Poster P010.
- Allen K, Pittock SJ et al. Sensitivity analysis using propensity score methods for primary efficacy outcome in the CHAMPION-NMOSD trial. Poster Presentation at: European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) Congress, October 26, 2022; Poster P012.
- Wingerchuk DM, Hogancamp WF, O’Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic’s syndrome). Neurology. 1999;53(5):1107-1114.
- Papadopoulos MC, Bennett JL, Verkman AS. Treatment of neuromyelitis optica: state-of-the-art and emerging therapies. Nat Rev Neurol. 2014;10(9):493.
- Cossburn, M., et al. (2012). The Prevalence of Neuromyelitis Optica in South East Wales." Eur J Neurol., 19(4): 655-659.
- Takata K, Matsuzaki T, Tajika Y. Aquaporins: water channel proteins of the cell membrane. Prog Histochem Cytochem. 2004;39(1):1-83.
- Mori M, Kuwabara S, Paul F. Worldwide prevalence of neuromyelitis optica spectrum disorders. J Neurol Neurosurg Psychiatry. 2018 Jun;89(6):555-556. doi: 10.1136/jnnp-2017-317566. Epub 2018 Feb 7. PMID: 29436488.
- Quek AML, Mckeon A, Lennon VA et al. Effects of age and sex on aquaporin-4 autoimmunity. Arch Neurol 2012 and 69:1039–43.
- Tüzün E, Kürtüncü M, Türkoğlu R, et al. Enhanced complement consumption in neuromyelitis optica and Behcet’s disease patients. J Neuroimmunol. 2011;233(1-2):211-215.
- Kuroda H, Fujihara K, Takano R, et al. Increase of complement fragment C5a in cerebrospinal fluid during exacerbation of neuromyelitis optica. J Neuroimmunol. 2013;254(1-2):178-182.
- Jarius, S., Wildemann, B. (2013). The History of Neuromyelitis Optica. J Neuroinflammation 10, 797.
- Mealy, M. A., et al. (2019). Assessment of Patients with Neuromyelitis Optica Spectrum Disorder Using the EQ-5D. International journal of MS care, 21(3), 129–134.
- ClinicalTrials.gov. An Efficacy and Safety Study of Ravulizumab in Adult Participants With NMOSD. NCT Identifier: NCT04201262. Available online. Accessed September 2022.
Dr. Pittock has provided consulting services to Alexion.
View source version on businesswire.com: https://www.businesswire.com/news/home/20221027005147/en/
Contacts
Media Inquiries
Todd Siesky +1 475 434 8140
Alexion Media Mailbox: media@alexion.com