MUNICH & SYDNEY - April 13, 2022 - PRLog -- OncoBeta® GmbH has announced its first Sydney-based patients were treated with Rhenium-SCT® as part of the global phase IV EPIC-Skin Study (Efficacy of Personalised Irradiation with Rhenium-SCT – for the treatment of non-melanoma skin cancer [NMSC]).
These patients were treated on 8 April and are now part of 210 adults participating in the international study that will follow their progress over the next 24 months.
The EPIC-Skin study is being conducted through study centres located in Australia, Austria, Germany and the United Kingdom. Hosting multiple study centres in Australia, these Sydney patients follow the Gold Coast patients that were the first-globally to be treated as part of the study.
Dr Sam Vohra, Medical Director at OncoBeta Australia, says, "Rhenium-SCT can be applied directly to an affected area, without harming or scarring surrounding tissue. It is important we collect data through the EPIC-Skin Study to further validate the efficacy, safety and patient quality of life after treatment of NMSCs with Rhenium-SCT."
There are more than 7.7 million cases of NMSC each year, and incidence rates are increasing globally.1,2 Standard treatments for NMSCs are surgery-based approaches, which may have a risk of scarring or loss of function. Rhenium-SCT uses a paste containing ß-emitting particles that is applied directly to the lesion, which eliminate cancer cells in one single session† and without the need for surgery.3-5
The EPIC-Skin study will measure Patient Reported Outcomes such as quality of life, treatment comfort and cosmetic outcomes, as well as further evaluating the efficacy of Rhenium-SCT for the treatment of NMSC. To provide a simple and streamlined way to record their experiences, patients in the study will utilise OncoBeta's Clinical Study app.
OncoBeta Australia Country Manager Ken Rikard-Bell says, "NMSC is a significant health concern both here in Australia and around the world. This study will offer new insights into the treatment of NMSC and the role of Rhenium-SCT® in the suite of treatments available to patients. It is exciting what this study could mean for the future of NMSC treatment."
Shannon D. Brown III, CEO and Managing Director at OncoBeta, says, "OncoBeta's goal is to provide the best innovative solutions for patients suffering from NMSCs. The patient journey is sometimes a difficult one and as a healthcare service provider we are committed to making this journey as easy, effective and efficient as possible. The EPIC-Skin Study will be critical in assisting us in improving patient outcomes for those suffering from NMSCs."
About the Rhenium-SCT® (Skin Cancer Therapy)
Non-melanoma skin cancer (NMSC) is the most common form of cancer in humans.2 The most common cause of NMSC is sun exposure, while other predisposing factors include genetic skin conditions and immunosuppressive diseases or treatments.6
The Rhenium-SCT® is a painless*, single session†, non-invasive therapy providing for unparalleled aesthetic results, even in cases otherwise considered difficult to treat.3-5 The Rhenium-SCT utilizes the radioisotope Rhenium-188 in an epidermal application with optimal properties for the treatment of NMSCs (non-melanoma skin cancers). The Rhenium-SCT is a precise, personalised therapy that is only applied to the area needed to treat without affecting the healthy tissue. The specially designed device ensures the Rhenium-SCT compound never comes in direct contact with the patient's skin and the application is safe and simple for the applying physician. Most cases of NMSCs (Basal Cell Carcinomas and Squamous Cell Carcinomas) can be treated using the Rhenium-SCT in one single session.†5 Scar-free healing of the treated lesion area and the regeneration of healthy tissue occurs usually within a few weeks after treatment.5
About OncoBeta®
OncoBeta®, with its headquarters located in Garching near Munich, Germany, is a privately held medical device company, specializing in the development and commercialization of state-of-the-art, innovative therapies. Since its foundation, OncoBeta has concentrated its efforts on the development, regulatory approval(s) and commercialization of the epidermal radioisotope therapy Rhenium-SCT® (Skin Cancer Therapy), targeting NMSCs. OncoBeta has perfected the customized application and device management system in conformity with all health, safety and environmental protection regulatory standards.
Find out more about the Rhenium-SCT® at www.oncobeta.com
Follow us on social media:
LinkedIn: www.linkedin.com/company/oncobeta-gmbh/
Facebook: www.facebook.com/oncobeta/
Instagram: www.instagram.com/oncobeta_gmbh/
Forward-looking statements
This announcement includes forward-looking statements that involve risks, uncertainties and other factors, many of which are outside of OncoBeta's control, and which could cause actual results to differ materially from the results discussed in the forward-looking statements. Forward-looking statements include statements concerning OncoBeta's plans, objectives, goals, future events, performance and/or other information that is not historical information. All such forward-looking statements are expressly qualified by these cautionary statements and any other cautionary statements which may accompany the forward-looking statements. OncoBeta® undertakes no obligation to publicly update or revise forward-looking statements to reflect subsequent events or circumstances after the date made, except as required by law.
*No reported pain3,4
†Complete tumour regression in 98.5% of lesions treated, with 89% after a single application5
References
- Global Burden of Disease Cancer Collaboration, et al. JAMA Oncol. 2019;5(12):1749-1768.
- Ciążyńska M, et al. Sci Rep. 2021;11(1):4337.
- Cipriani C, et al. J Dermatolog Treat. 2020; Jul 22:1-7.
- Sedda AF, et al. Clin Exp Dermatol. 2008;33(6):745-749.
- Cipriani C, Sedda AF. Epidermal Radionuclide Therapy - Dermatological High-Dose-Rate. Brachytherapy for the Treatment of Basal and Squamous Cell Carcinoma. In: Therapeutic Nuclear Medicine, editor Baum RP; New York: Springer, 2014.
- Cancer.net. Skin Cancer (Non-Melanoma): Risk Factors and Prevention. October 2020. https://www.cancer.net/cancer-types/skin-cancer-non-melanoma/risk-factors-and-prevention (accessed November 2021).
Contact
Jane Morey
***@moreymedia.com.au
Photos: (Click photo to enlarge)
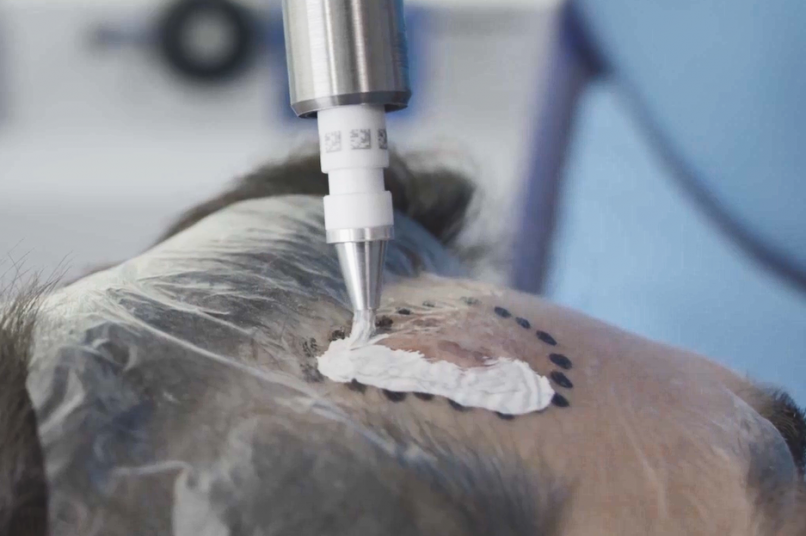

Read Full Story - Sydney skin cancer patients join the EPIC-Skin study as they undergo first treatments with OncoBeta's Rhenium-SCT | More news from this source
Press release distribution by PRLog