Theralase®'s Latest Clinical Data Demonstrates 65% of Patients Treated with Theralase®'s Anti-Cancer Therapy Achieved a Complete Response
TORONTO, ON / ACCESSWIRE / October 16, 2023 / Theralase® Technologies Inc. ("Theralase®" or the "Company") (TSXV:TLT)(OTCQB:TLTFF) is a clinical-stage pharmaceutical company dedicated to the research and development of light or radiation activated Photo Dynamic Compounds ("PDCs") for the safe and effective destruction of various cancers, bacteria and viruses.
Theralase® is providing an update to its Phase II Bacillus Calmette Guérin ("BCG")-Unresponsive Non-Muscle Invasive Bladder Cancer ("NMIBC") Carcinoma In-Situ ("CIS") (with or without resected Ta / T1 papillary disease) clinical study ("Study II").
To date, Theralase® has enrolled and provided the primary Study II Treatment for 62 patients.
The Study II Endpoints have been defined as:
Primary: Efficacy - Defined as Complete Response ("CR") rate at any point in time (CR is defined as at least one of the following:
- Negative cystoscopy and negative (including atypical) urine cytology
- Positive cystoscopy with biopsy-proven benign or low-grade NMIBC and negative cytology
- Negative cystoscopy with malignant urine cytology, if urothelial cancer is suspected in the upper tract or prostatic urethra and random bladder biopsies are negative)
Secondary: Duration of CR - Defined as 12 months post initial CR assessment
Tertiary: Safety - Defined as the incidence and severity of Adverse Events ("AEs"), Grade 4 or higher, directly related to the Study Drug or Study Device, that do not resolve within 450 days post primary Study II Treatment; whereby: Grade 1 = Mild, Grade 2 = Moderate, Grade 3 = Severe, Grade 4 = Life-threatening or disabling, Grade 5 = Death.
In addition, survival surveillance for all patients, who achieve a CR or Indeterminate Response ("IR") (negative cystoscopy and positive urine cytology, without confirmatory negative bladder cancer biopsies) at 450 days and remain in Study II will be monitored past 450 days.
In performance to the primary, secondary and tertiary endpoints, refer to the clinical data below.
Achieved Primary Objective (n) |
Achieved Primary Objective (%) |
Achieved Secondary Objective (n) |
Achieved Secondary Objective (%) |
Achieved Tertiary Objective (n) |
Achieved Tertiary Objective (%) |
|
Complete Response ("CR") |
37 |
65% |
14 |
33% |
57 |
100% |
Indeterminate Response ("IR") |
5 |
9% |
2 |
5% |
0 |
0% |
Total Response (CR and IR) |
42 |
74% |
16 |
38% |
57 |
100% |
Evaluable Patients |
57 |
42 |
57 |
Note: Evaluable patients are defined as patients who have been assessed by a principal investigator during a particular assessment visit.
The CR, IR and Total Responders are detailed below by assessment visit.
TLD 1433-2 Clinical Study (Evaluable Patients) | ||||||||||
Assessment |
90 Day |
90 Day |
180 Day |
180 Day |
270 Day |
270 Day |
360 Day |
360 Day |
450 Day |
450 Day |
# |
% |
# |
% |
# |
% |
# |
% |
# |
% |
|
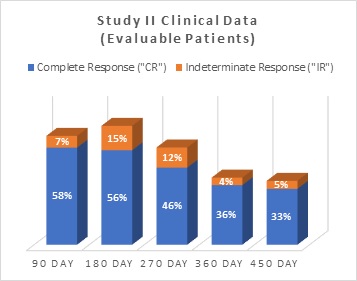
Complete Response ("CR") |
33 |
58% |
31 |
56% |
23 |
46% |
16 |
36% |
14 |
33% |
Indeterminate Response ("IR") |
4 |
7% |
8 |
15% |
6 |
12% |
2 |
4% |
2 |
5% |
Total Responders (CR and IR) |
37 |
65% |
39 |
71% |
29 |
58% |
18 |
40% |
16 |
38% |
Total Evaluated |
57 |
55 |
50 |
45 |
42 |
|||||
On August 1, 2020, the Company optimized the Study II Treatment. For patients that received the optimized Study II Treatment the CR, IR and Total Responders are detailed below by assessment visit.
TLD 1433-2 Clinical Study (Evaluable Patients) (Optimized: Post August 1, 2020) | |||||||||||
Assessment |
90 Day |
90 Day |
180 Day |
180 Day |
270 Day |
270 Day |
360 Day |
360 Day |
450 Day |
450 Day |
|
# |
% |
# |
% |
# |
% |
# |
% |
# |
% |
||
Complete Response ("CR") |
29 |
64% |
28 |
62% |
20 |
50% |
13 |
37% |
11 |
34% |
|
Indeterminate Response ("IR") |
3 |
7% |
7 |
16% |
5 |
13% |
2 |
6% |
2 |
6% |
|
Total Responders (CR and IR) |
32 |
71% |
35 |
78% |
25 |
63% |
15 |
43% |
13 |
41% |
|
Total Evaluated |
45 |
45 |
40 |
35 |
32 |
||||||
Theralase® is currently working with its clinical study sites in Canada and the United States to compile information requested by the Food and Drug Administration ("FDA") for re-submission of a pre-Break Through Designation ("BTD").
If the pre-BTD submission is successful, this could lead to BTD approval.
Arkady Mandel, MD, PhD, DSc, Chief Scientific Officer of Theralase® stated, "Theralase® is delighted in its latest clinical data analysis. The Theralase® RuvidarTM-based Anti-Cancer Therapy ("ACT") has shown remarkable single-agent activity by proving to be safe and effective on a very difficult to treat BCG-Unresponsive patient population that has been diagnosed with high-grade NMIBC CIS, with or without resected Ta / T1 papillary tumours. These patients have failed the standard of care, such as BCG therapy and a large majority of them have failed treatment with various modern immunotherapy drugs. Theralase® has been able to demonstrate strong efficacy in the form of a CR or IR, with a well-tolerated safety profile, after predominately one treatment. This ACT technology, pending successful regulatory approval and commercialization, will be very attractive to patients, uro-oncologists and the insurance companies that insure these patients."
Mr. Roger DuMoulin-White, BSc, P.Eng, Pro.Dir, President and Chief Executive Officer of Theralase® stated, "To date, the Theralase® Study II clinical data has demonstrated best-in-class performance for a single agent, providing high efficacy, durable response and a high safety profile, with no serious adverse events directly related to RuvidarTM or the TLC-3200 Medical Laser System. Theralase® hopes to complete patient enrollment with accompanying administration of the primary Study II Treatment by year end 2024. If successful, this will allow the Company the ability to complete assessment of the primary, secondary and tertiary endpoints of these patients by 2026. Based on the clinical data to date, Theralase® is investigating potential partnerships for commercialization, financing and distribution of this ACT technology on an international basis. Theralase® looks forward to commercializing this world-class technology for the benefit of all shareholders."
About Theralase® Technologies Inc.
Theralase®, a clinical stage pharmaceutical company dedicated to the research and development of Photo Dynamic Compounds ("PDCs"), in addition to the light and radiation systems that activate them, is focused on the safe and effective destruction of various cancers, bacteria and viruses, when light or radiation activated.
Additional information is available at www.theralase.com and www.sedar.com
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
Forward-Looking Statements
This news release contains Forward-Looking Statements ("FLS") within the meaning of applicable Canadian securities laws. Such statements include, but are not limited to, statements regarding the Company's proposed development plans with respect to Photo Dynamic Compounds ("PDCs") and their drug formulations. FLS may be identified by the use of the words "may, "should", "will", "anticipates", "believes", "plans", "expects", "estimate", "potential for" and similar expressions; including, statements related to the current expectations of the Company's management for future research, development and commercialization of the Company's PDCs and their drug formulations; including: preclinical research, clinical studies and development and regulatory approvals.
These statements involve significant risks, uncertainties and assumptions; including, whether the Company is able to: adequately fund and secure the requisite regulatory approvals to successfully complete preclinical and clinical studies in a timely fashion to implement its development plan; successfully commercialize its drug formulations; access sufficient capital to fund the Company's operations, which may not be available on terms that are commercially favorable to the Company or at all; provide preclinical and clinical support that the Company's drug formulations are effective against the conditions tested in its preclinical and clinical studies; comply with the term of license agreements with third parties, not to lose the right to use key intellectual property in its business; protect its intellectual property and the timing and success of this intellectual property and achieve acceptance and approval of regulatory filings. Many of these factors that will determine actual results are beyond the Company's ability to control or predict.
Readers should not unduly rely on these FLS, which are not a guarantee of future performance. There can be no assurance that FLS will successfully come to fruition, and as such, FLS involve known and unknown risks, uncertainties and other factors which may cause actual results or future events to differ materially from the FLS.
Although the FLS contained in the press release are based upon what management currently believes to be reasonable assumptions, the Company cannot assure prospective investors that actual results, performance or achievements will be consistent with these FLS.
All FLS are made as of the date hereof and are subject to change. Except as required by law, the Company assumes no obligation to update such statements.
For More Information:
1.866.THE.LASE (843.5273)
416.699.LASE (5273)
www.theralase.com
Kristina Hachey, CPA
Chief Financial Officer
khachey@theralase.com
416.699.LASE (5273) x 224
SOURCE: Theralase Technologies Inc.
View source version on accesswire.com:
https://www.accesswire.com/793007/theralaser-provides-update-on-bladder-cancer-clinical-study