ACON Laboratories Announces Breakthrough 4-in-1 Flowflex® Plus RSV + Flu A/B + COVID Home Test
October 28, 2025 at 03:00 AM EDT
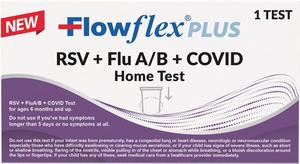
Single-Swab Solution: New Over-the-Counter Test Simultaneously Detects and Differentiates RSV, Flu A/B, and COVID from the Comfort of Home
SAN DIEGO, CA, October 28, 2025 /24-7PressRelease/ -- ACON Laboratories, Inc., a leading global medical device manufacturer, announced today that its Flowflex® Plus RSV + Flu A/B + COVID Home Test has received 510(k) clearance from the U.S. Food & Drug Administration (FDA). The Flowflex Plus RSV + Flu A/B + COVID Home Test (K251749) is an over-the-counter (OTC) rapid antigen test that can be administered in the comfort and privacy of consumers' homes. This new test will be manufactured domestically in ACON's state-of-the-art facility in San Diego, CA.
The Flowflex Plus RSV + Flu A/B + COVID Home Test is a lateral flow immunoassay intended for the rapid, simultaneous qualitative detection and differentiation of respiratory syncytial virus (RSV), influenza A, influenza B (Flu A/B), and SARS-CoV-2 (COVID) protein antigens. This test is authorized for non-prescription home use with self-collected anterior nares nasal swab specimens. This is not only the first device of its kind with four analytes in one test (4-in-1), and the first RSV home test, but it is also the first respiratory home test to earn FDA clearance for children aged 6-23 months when administered by an adult using ACON's proprietary nasal swab guard.
In summary, this innovative product is:
- The first FDA-cleared 4-in-1 respiratory home test
- The first FDA-cleared RSV home test
- The first FDA-cleared respiratory infection home test for children aged 6-23 months
"We are excited to introduce this game-changing 4-in-1 home test. This product further demonstrates our commitment to enabling consumers to take charge of their own health. Symptoms of respiratory infections can be similar, so knowing which infection you have is important for making early treatment decisions, which can lead to better outcomes. We expect the Flowflex 4-in-1 home test will bring peace of mind to parents of young children as well as the elderly, the immune-compromised, and those of us who are in contact with at-risk populations," commented VP of Sales & Marketing, Michael Lynch.
Flowflex continues to be America's #1 home test brand¹, and the Flowflex Plus RSV + Flu A/B + COVID Home Test will be widely available at major retailers later this year. A list of authorized distributors and retail partners can be found at www.flowflexcovid.com.
¹ SOURCE: Circana Retail Sales Data (Units sold)
---
Press release service and press release distribution provided by https://www.24-7pressrelease.com
More News
View More
3 Recently Downgraded Stocks to Avoid in 2026 ↗
December 11, 2025
Via MarketBeat
The Chip Boom Is Back: 3 Stocks Positioned for Huge Gains ↗
December 11, 2025

Oracle Stock Hit Hard: Why Its AI Pipeline Could Drive a 2026 Rally ↗
December 11, 2025

Beyond the Magnificent 7: Meet 3 of Tech’s Rising Stars ↗
December 11, 2025

The Quantum Fleet: Investing in the New Quantum Standard ↗
December 11, 2025
Recent Quotes
View More
Stock Quote API & Stock News API supplied by www.cloudquote.io
Quotes delayed at least 20 minutes.
By accessing this page, you agree to the Privacy Policy and Terms Of Service.
Quotes delayed at least 20 minutes.
By accessing this page, you agree to the Privacy Policy and Terms Of Service.
© 2025 FinancialContent. All rights reserved.
>