Open Doors to the Asian Market: Seoul Bio Hub-Celltrion Launches ‘Global Open Innovation’ for U.S. Startups
Seoul Bio Hub and Celltrion Launch Strategic Gateway ‘Global Open Innovation’ to Help U.S. Bio Startups Dominate the Asian Market
Two U.S.-Based Biotech and Medical Device Startups to Be Selected Based on Technology Alignment with Celltrion’s Expertise in Antibodies, Peptides, and Drug Formulation
Seoul, Korea – 17/09/2025 – (SeaPRwire) – Seoul’s bio startup hub ‘Seoul Bio Hub’ and global pharmaceutical leader ‘Celltrion’ have joined forces to launch the ‘2025 Seoul Bio Hub-Celltrion Global Open Innovation (GOI)’ program. This program goes beyond a simple competition, serving as a platform designed to help U.S. startups successfully establish themselves in the Asian market.
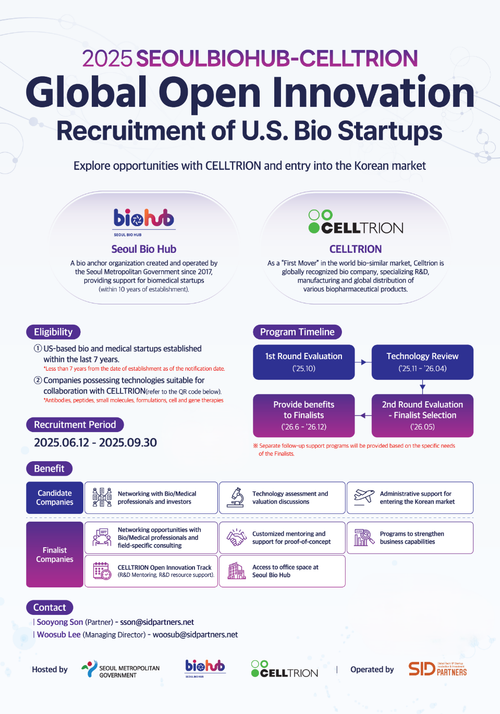
This GOI targets U.S.-based bio and medical startups, with companies established within the past 7 years eligible to apply. The recruitment areas encompass all technologies suitable for collaboration with Celltrion, including antibodies, peptides, small molecules, formulations, and cell and gene therapies.
The application deadline is September 30, 2025. The selection process will proceed in the following order:
- Primary document evaluation
- Technology review
- Secondary presentation evaluation.
The two finally selected companies will receive customized follow-up support and gain opportunities to expand into global markets through collaboration with Celltrion, leveraging South Korea’s innovative ecosystem and global capabilities.
A Seoul Bio Hub representative stated, “This program will serve as a bridgehead that goes beyond simple space support, enabling U.S. startups to rapidly grow in the Asian market by leveraging Seoul’s excellent clinical infrastructure and research environment.”
A Celltrion representative announced, “Based on Celltrion’s capabilities and know-how accumulated across the entire pharmaceutical industry process including R&D, production, and sales, we will support promising U.S. startups to successfully enter the Asian market and create synergy with our company.”
South Korea has established itself as a bio hub leading the global market with clear numerical evidence. According to global consulting firm Intralink, South Korea’s biopharmaceutical market is valued at approximately $22 billion, ranking 13th globally.
Additionally, market research firm Grand View Research forecasts that South Korea’s biotechnology market will grow to $81.6 billion (approximately 110 trillion KRW) by 2030, representing an annual average growth rate of 18.3%.
Private investment is also active. From 2020 to 2022, R&D and facility investments recorded an annual average growth rate of 21.6%. This demonstrates that South Korea is not merely a potential market, but a rapidly growing innovation ecosystem.
According to ClinicalTrials.gov, the global clinical trial database, Seoul has consistently maintained a top position among major global cities in the number of new clinical trials over the past decade.
South Korea’s Ministry of Health and Welfare has set ‘Achieving Global 3rd Place in Clinical Trials’ as a policy objective through the ‘3rd Five-Year Plan for the Pharmaceutical and Bio Industry (2023-2027)’. Furthermore, in 2025, it raised a total of over 386.6 billion KRW through the ‘Bio Health Mega Fund’ and is actively supporting innovative companies’ clinical trials and commercialization through the national integrated bio big data construction project and AI, bio health regulatory regulatory sandboxes.
Seoul Bio Hub is a bio startup support organization established by Seoul City in 2017. It provides 251 pieces of shared laboratory equipment and affordable tenant space at 10% of the cost compared to major areas in Seoul. As of May 2025, it has supported 322 startups and attracted a total of 576 billion KRW in follow-up investment, proving its potential for success.
Celltrion is South Korea’s global comprehensive biotechnology company that developed the world’s first antibody biosimilar ‘Remsima’. The company performs the entire process from research and development to approval, production, and sales, supplying high-quality biopharmaceuticals to over 100 countries worldwide. Based on world-class research and production infrastructure, it contributes to expanding global access to pharmaceuticals and continues to support and collaborate with promising startups through active open innovation strategies.
For detailed information regarding program applications and inquiries, please access LinkedIn, search for ‘Seoul Bio Hub’, and check the 「2025 Seoul Bio Hub-Celltrion Global Open Innovation」 recruitment announcement posted on the official company page.
About Seoul Bio Hub
https://www.seoulbiohub.kr/front/user/engmain.do
Establishment Date: October 30, 2017
Operating Entity: Established by Seoul City / Joint operation by KIST and Korea University
Location: 117-3 Hoegi-ro, Dongdaemun-gu, Seoul, Republic of Korea
Purpose: Growth and commercialization support for early-stage bio and healthcare startups under 10 years
Tenant Companies (As of June 2025): Total 126 companies (Digital Health 42 companies (33.3%), Pharmaceuticals 61 companies (48.5%), Medical Devices 23 companies (18.2%))
Infrastructure: Laboratories, office spaces, meeting rooms, conference rooms, research equipment (249 devices, 109 types, approximately $8 million scale)
Research and Human Resource Network (Regional): Approximately 7,000 PhD-level personnel, approximately 120,000 university students, $1.13 billion in research funding, connections with 12 universities and research institutions and 7 hospitals
Major Support Programs:
- IR matching and investment linkage
- Expert consulting and CEO education
- Hospital-linked clinical and data programs
- Open innovation with domestic and international pharmaceutical companies
About Celltrion
https://www.celltrion.com/en-us
Establishment Date: February 26, 2002
Location (Headquarters): 23 Academy-ro, Yeonsu-gu, Incheon Metropolitan City, Republic of Korea
Business Areas and Major Products (Services)
Possessing Differentiated Integrated Solutions Across the Entire Biopharmaceutical Business Process
- Remsima: World’s first monoclonal antibody biosimilar (TNF-α inhibitor)
- Herzuma: Trastuzumab biosimilar for breast and gastric cancer treatment
- Truxima (CT-P10): Rituximab biosimilar (lymphoma, rheumatoid arthritis, etc.)
- Multiple products including Zymfentra, Yuflyma, Vegzelma, Steqeyma, Avtozma are under global approval and commercialization
No of Employees: Approx. 3,003 (As of June 30, 2025)
Media Contact
Brand: SEOUL BIO HUB
Contact: Lee Yoon Jin
Phone: +82 02-2200-3345
Email: lynj@kist.re.kr
Website: (KOR) https://www.seoulbiohub.kr/front/user/main.do;
More News
View MoreRecent Quotes
View More
Quotes delayed at least 20 minutes.
By accessing this page, you agree to the Privacy Policy and Terms Of Service.




