Clinical Evidence Shows Reduced Costs and Improved Outcomes in Heart Transplantation
ASAIO Journal publishes a new clinical study illustrating that Paragonix advanced cardiac preservation technology correlates with a significant reduction in post-transplant complications and average hospital expenses compared to conventional ice storage
Paragonix Technologies, Inc., a leader in organ preservation technology and research, has announced the publication of a groundbreaking organ transplant study in the American Society for Artificial Internal Organs (ASAIO) Journal. This new study utilizes real-world clinical data to contrast two preservation methods: the Paragonix SherpaPak® Cardiac Transport System (PGNX), and the historic method of organ transport, ice cold storage (ICE). The study illustrates the favorable post-operative impact that advanced preservation technology may have on hospital costs and patient outcomes after heart transplant procedures.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20221122005269/en/
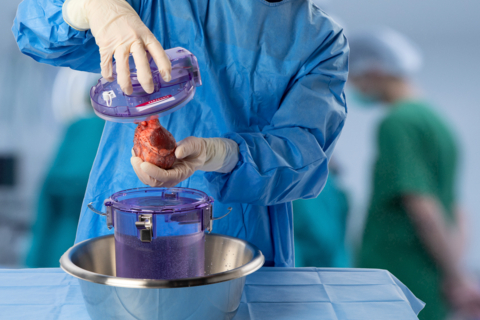
Paragonix SherpaPak® Cardiac Transport System (CTS) has preserved more than 2,200 hearts since start of clinical use in 2018. The leading FDA cleared and CE marked preservation device for heart transportation, Paragonix SherpaPak CTS provides a sterile, temperature and pressure-controlled environment for organs traveling between operating rooms. (Photo: Business Wire)
The study’s full dataset was collected from August 2015 to November 2021 as part of the GUARDIAN-Heart Clinical Registry, analyzing a total of 174 propensity-matched patients (87 PGNX and 87 ICE) from 12 transplant hospitals across the United States. The results of the study indicate that patients who had their hearts transported utilizing ice cold storage spent a significantly higher portion of their ICU days on mechanical circulatory support (MCS) when compared to patients whose donor hearts were preserved with the Paragonix SherpaPak System (40.3% [ICE] vs. 10.8% [PGNX], p=<0.0001). Upon further comparison of the preservation methods, the transplants which utilized the Paragonix SherpaPak System displayed a statistically significant reduction of $26,700 in average hospital costs, due to the favorable clinical impacts affecting cost variables such as MCS usage, ICU stay, and overall hospital stay.
The Paragonix SherpaPak Cardiac Transport System is an FDA-cleared and CE-marked device intended specifically to transport, preserve, and monitor donor hearts traveling from donor to recipient. The analysis shows that when the advanced preservation device was used, recipients experienced a statistically significant reduction in the rate of severe Primary Graft Dysfunction (PGD) (16.1% [ICE] vs. 5.7% [PGNX], p=0.03). The study also found a reduced need for ECMO and usage of temporary VAD when comparing the patient cohort that utilized the Paragonix SherpaPak to the cohort reliant on cold ice storage.
“The adoption of innovative technology in the realm of transplantation marks a turning point for an industry that has remained unchanged for decades. In this study, patients in the SherpaPak cohort spent fewer days on mechanical circulatory support in ICU which resulted in a statistically significant reduction in total cost per patient,” said Dr. David D'Alessandro, Surgical Director, Heart Transplantation and Ventricular Assist Devices, Member of Faculty at Harvard Medical School, and a U.S. principal investigator of the GUARDIAN-Heart study.
The study concluded that the usage of the Paragonix SherpaPak System within the United States displays potential benefits in terms of improving clinical outcomes and hospital costs when compared directly to traditional ice cooler methods of transport. “I believe it is critical to highlight the superior post-transplant outcomes associated with lowering the rate of severe primary graft dysfunction, as well as the utilization of mechanical circulatory support after a heart procedure,” said Dr. Yasuhiro Shudo, a cardiothoracic surgeon and clinical assistant professor in the Department of Cardiothoracic Surgery at Stanford University School of Medicine. “In this study, the results indicate that the Paragonix SherpaPak can potentially improve the post-transplant outcomes of patients, leading to lowered overall costs for hospitals.”
“Advanced organ preservation devices have become the new standard of care for patients,” said Dr. Lisa Anderson, President and CEO of Paragonix Technologies. “Our technology continues to operate in top hospitals and transplant centers across the country. The data shows that advanced preservation technology is a cost-effective and lower-risk solution than traditional transportation methods.”
About Paragonix Technologies
Paragonix Technologies is a leading provider of Advanced Organ Preservation (AOP) devices that safeguard donor organs during the journey between donor and recipient patients. Our FDA-cleared and CE-marked devices incorporate clinically proven and medically trusted cold preservation techniques that allow unprecedented physical and thermal protection to the organ in transit. Every Paragonix AOP device natively integrates with our novel digital app, delivering real-time organ tracking data and monitoring logistics for transplant teams seeking a secure and centralized solution.
GUARDIAN is a registered clinical study (NCT04141605) funded and administered by Paragonix Technologies. At the time of this analysis, GUARDIAN contained data from 12 sites on 490 patients. The data from the registry is descriptive, not statistically powered, and not pre-specified. The information should be interpreted accordingly. In this analysis, US adult cohorts were matched using statistical propensity matching to create cohorts of equal baseline characteristics (87 ice transports and 87 Paragonix SherpaPak CTS transports.
Connect with us on LinkedIn: Paragonix Technologies
Follow us on Twitter: @ParagonixSherpa
Like Us on Facebook: Paragonix SherpaPak
L-404 Rev. 0
View source version on businesswire.com: https://www.businesswire.com/news/home/20221122005269/en/
Contacts
Media:
Adam Lafreniere
Sr. Director, Marketing
marketing@paragonixtechnologies.com
More News
View More


Recent Quotes
View More
Quotes delayed at least 20 minutes.
By accessing this page, you agree to the Privacy Policy and Terms Of Service.