Pliant Therapeutics Announces Interim Phase 1 Data for PLN-101095 in Patients with Immune Checkpoint Inhibitor-Refractory Advanced Solid Tumors
Antitumor activity observed with confirmed partial responses in 50% of patients
at highest dose tested to date, across multiple tumor types
PLN-101095 was generally well tolerated across all doses
SOUTH SAN FRANCISCO, Calif., March 17, 2025 (GLOBE NEWSWIRE) -- Pliant Therapeutics, Inc. (Nasdaq: PLRX) today announced data from the first three of five potential cohorts of its ongoing Phase 1 dose escalation clinical trial evaluating PLN-101095, an integrin αvβ8 and αvβ1 inhibitor, in combination with pembrolizumab, in patients with immune checkpoint inhibitor (ICI) -refractory advanced or metastatic solid tumors. Interim results demonstrated PLN-101095 anti-tumor activity in combination with pembrolizumab, with three partial responses observed in cohort three at the 1000 mg administered orally twice daily (BID) dose, representing a 50% objective response rate (ORR) at the highest dose tested to date. PLN-101095 was generally well tolerated across all doses tested.
Nine patients with six different tumor types were enrolled in cohorts one through three of the trial. Patients were treated for 14 days with PLN-101095 at doses of 250 mg, 500 mg or 1000 mg administered orally BID, followed by treatment with a combination of PLN-101095 and pembrolizumab at 200 mg administered intravenously every three weeks (Q3W). Scans were conducted at baseline, Day 14, Week 10, and every 8 weeks thereafter.
The Company reported the following initial observations from the trial:
- Across all doses tested, PLN-101095 was generally well tolerated
- Of the six patients treated at the 1000 mg BID dose of PLN-101095, three (50%) confirmed partial responses were observed. All three patients remain on treatment.
- Non-Small Cell Lung Cancer (NSCLC): Confirmed partial response with a 74% reduction in tumor size at Week 18; initial partial response was observed at Week 10
- Cholangiocarcinoma: Confirmed partial response with a 48% reduction in tumor size at Week 42; initial partial response was observed at Week 34
- Melanoma: Confirmed partial response with a 42% reduction in tumor size at Week 27; initial partial response was observed at Week 18
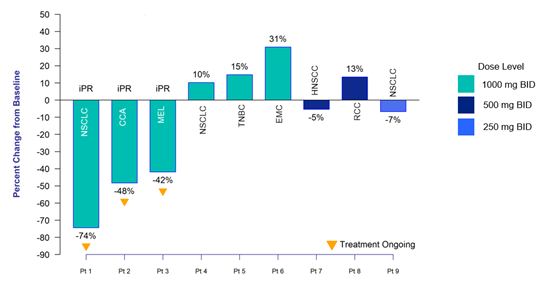
iPR: confirmed partial response (>30% reduction in Baseline target lesions)
CCA: cholangiocarcinoma; EMC: endrometrial cancer; HNSCC: head and neck squamous cell carcinoma; MEL: melanoma; NSCLC: non-small cell lung cancer; RCC: renal cell carcinoma; TNBC: triple negative breast cancer
Figure 1. Maximum (+, -) Percent Change from Baseline in Tumor Size
“We are encouraged by these early responses given the refractory nature of the patient population enrolled in this trial,” said Éric Lefebvre, M.D., Chief Medical Officer of Pliant. “We look forward to sharing the final results from this trial in the future.”
The Phase 1 trial of PLN-101095 is currently enrolling the fourth of five potential cohorts. The fourth cohort is evaluating PLN-101095 at 1000 mg, three times daily (TID).
Slides accompanying these data can be found here and under the Investors & Media page of the Pliant website at www.PliantRx.com.
PLN-101095 for Treatment of Checkpoint Resistant Tumors
PLN-101095 is an oral small molecule inhibitor of integrins αvβ8 and αvβ1. It is currently being evaluated in an ongoing first-in human Phase 1 dose-escalation trial. The open-label trial (NCT06270706) is designed to evaluate the safety, tolerability, pharmacokinetics, pharmacodynamics and preliminary antitumor activity of PLN-101095 alone and in combination with the immunotherapy pembrolizumab. Activated transforming growth factor-β (TGF-β) has shown to foster an immuno-suppressive tumor microenvironment (TME) that contributes to immune-checkpoint inhibitor (ICI) resistance and treatment failure in cancer.1 Blocking integrins αvβ8 and αvβ1 has shown to prevent the activation of TGF-β and is expected stimulate immune activation by increasing immune cell infiltration into the tumor microenvironment.2,3 In preclinical studies, PLN-101095 demonstrated monotherapy activity in reduction of tumor volume and increased cluster of differentiation (CD)8+ T cell infiltration. In addition, PLN-101095 in combination with an anti-PD-1 monoclonal antibody (mAb) elicited a dose-dependent reduction in tumor volume and increased CD8+ T cell tumor infiltration in the tumor microenvironment compared with anti-PD-1 mAb therapy alone.4
About Pliant Therapeutics, Inc.
Pliant Therapeutics is a late-stage biopharmaceutical company and leader in the discovery and development of novel therapeutics for the treatment of fibrotic diseases. Pliant's lead product candidate, bexotegrast (PLN-74809), is an oral, small molecule, dual selective inhibitor of αvβ8and αvβ1 integrins that is in development in the lead indication for the treatment of idiopathic pulmonary fibrosis, or IPF. Bexotegrast has received Fast Track Designation and Orphan Drug Designation from the U.S. Food and Drug Administration (FDA) and Orphan Drug Designation from the European Medicines Agency in IPF. Pliant is conducting a Phase 1 study for PLN-101095, a small molecule, dual-selective inhibitor of αvβ8 and αvβ1 integrins, that is being developed for the treatment of solid tumors. In addition, Pliant has received regulatory clearance for the conduct of a Phase 1 study of PLN-101325, a monoclonal antibody agonist of integrin α7β1 targeting muscular dystrophies.
For additional information, please visit: www.PliantRx.com. Follow us on social media X, LinkedIn, and Facebook.
Forward-Looking Statements
Statements contained in this press release regarding matters that are not historical facts are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as "may," "will," "expect," "anticipate," "estimate," "intend," and similar expressions (as well as other words or expressions referencing future events, conditions, or circumstances) are intended to identify forward-looking statements. These statements include those regarding the potential benefits of PLN-101095 and the Company’s plans for the continued development of PLN-10195, as well as statements regarding the development of the Company’s other clinical and pipeline assets. Because such statements deal with future events and are based on our current expectations, they are subject to various risks and uncertainties and actual results, performance or achievements of Pliant Therapeutics could differ materially from those described in or implied by the statements in this press release. These forward-looking statements are subject to risks and uncertainties, including those related to the development and commercialization of our product candidates, including analysis of the complete data from the BEACON-IPF trial, any delays in our ongoing or planned preclinical or clinical trials, the risks inherent in the drug development process, and our capital requirements and the need for additional financing, including the anticipated lack of availability of additional funds under the current terms of our loan facility. These and additional risks are discussed in the sections titled "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" in our Quarterly Report on Form 10-Q for the period ended September 30, 2024, which is available on the SEC's website at www.sec.gov, as updated by our Annual Report on Form 10-K for the year ended December 31, 2024, which we expect to file with the SEC today. Unless otherwise noted, Pliant is providing this information as of the date of this news release and does not undertake any obligation to update any forward-looking statements contained in this document as a result of new information, future events or otherwise.
Investor and Media Contact:
Christopher Keenan
Vice President, Investor Relations and Corporate Communications
Pliant Therapeutics, Inc.
ir@pliantrx.com
1 Pickup M. et al. Nat Rev Cancer. 2013 Nov;13(11):788-99.
2 Takasaka N. et al. JCI Insight. 2018 Oct 18;3(20).
3 Reed NI. et al. Sci Transl Med. 2015 May 20;7(288):288ra79.
4 Kothari V, et al. J Immunother Cancer 2022;10(Suppl 2): A1403 abstract 1352 (SITC 2022)

More News
View More



Recent Quotes
View More
Quotes delayed at least 20 minutes.
By accessing this page, you agree to the Privacy Policy and Terms Of Service.
