HMNC Brain Health Announces Phase 2 Results from OLIVE Trial in Major Depressive Disorder (MDD) with Genetically Guided Precision Approach
BH-200 (nelivaptan) achieved clinically meaningful reduction in depressive symptoms across the full study population (Difference between BH-200 and placebo in HAM-D17 change from baseline at Week 8: -2.98; p = 0.0003)
The largest and most rapid improvement was observed in a genetically pre-defined subgroup representing 27% of patients (Difference between BH-200 and placebo in HAM-D17 change from baseline at Week 8: -4.47; p = 0.005)
Findings establish first-in-class potential for an HPA-axis modulator (HPAM) in depression treatment
Results underscore the potential of HMNC’s biomarker-guided approach, supporting a shift toward more precise, biology-driven treatment in MDD
MUNICH, Aug. 05, 2025 (GLOBE NEWSWIRE) -- HMNC Brain Health (“HMNC”), a clinical-stage precision psychiatry biopharmaceutical company, today announced topline results from its OLIVE trial, a randomized, double-blind, placebo-controlled Phase 2b trial evaluating BH-200 (nelivaptan), a selective vasopressin V1b receptor antagonist, in patients with Major Depressive Disorder (MDD).
Topline Results
The OLIVE trial evaluated the efficacy of BH-200, a highly selective vasopressin V1b receptor antagonist, which has demonstrated efficacy in a previous Phase 2 trial (N=319) in patients with MDD. The OLIVE trial enrolled 338 patients with MDD across eight countries in Europe between May 2023 and April 2025. Patients were randomized in a 2:1 ratio to receive BH-200 (250 mg BID, i.e., twice daily) or placebo for eight weeks, with the primary endpoint defined as mean HAM-D17 total score change from baseline to Week 8 in one of the three subgroups classified by the genetic selection tool (Subgroup C). The modified intention-to-treat (mITT) set comprised 331 patients, and 295 were completers.
The study demonstrated that treatment with BH-200 led to a clinically meaningful reduction in depressive symptoms from baseline to Week 8 across the full patient population (difference between BH-200 and placebo in HAM-D17: -2.98; SE = 0.82; p = 0.0003; Cohen’s d effect size = 0.44). Notably, a larger and more rapid effect was observed in a genetically defined subgroup — Subgroup A (n = 89), comprising 27% of patients — identified through HMNC’s proprietary genetic selection tool with a difference between BH-200 and placebo in HAM-D17 mean change from baseline at Week 8: -4.47; SE 1.58; p = 0.005; Cohen’s d effect size = 0.55.
Precision Psychiatry Breakthrough
A central innovation in the OLIVE study was HMNC’s proprietary genetic selection tool, which classifies patients based on biomarkers linked to vasopressin signaling and regulation of the stress-response system, the hypothalamic-pituitary-adrenal (HPA) axis. The tool classified patients into three biologically distinct subgroups based on vasopressin-related biology. While the study’s primary endpoint was pre-specified in Subgroup C, the findings suggest that lower peripheral vasopressin activity (Subgroup A) correlates with stronger central vasopressin activity in relevant brain areas, resulting in a more pronounced antidepressant response in Subgroup A. This confirms the hypothesis that a genetic test can guide patient selection for BH-200, marking a critical step toward precision-guided depression treatment.
Trial & Findings Details
Modified ITT (mITT) Population, N = 331
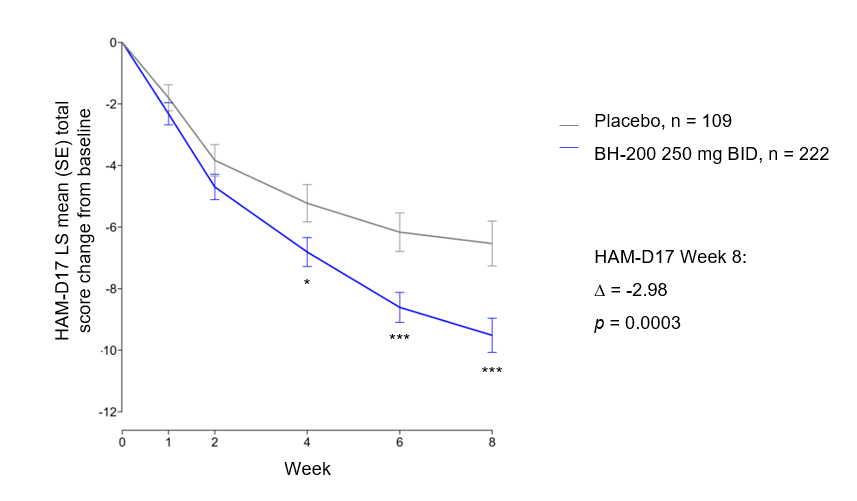
In the full modified intention-to-treat (mITT) population, BH-200 demonstrated a clinically meaningful improvement, with a separation from placebo on HAM-D17 mean change from baseline emerging at Week 4 (p < 0.05) and increasing over time. By Week 8, the mean difference versus placebo was -2.98 (SE 0.82; p = 0.0003).
The treatment effect across pre-defined genetic subgroups was evaluated by comparing the mean HAM-D17 change from baseline to Week 8 compared with the respective placebo group.
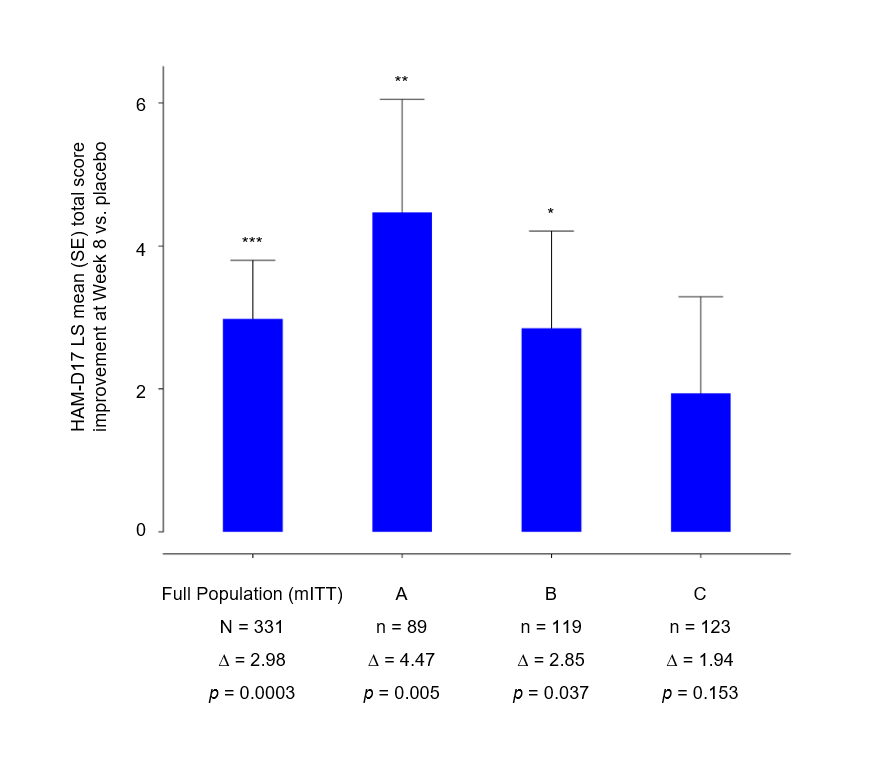
The data revealed a clinically meaningful improvement in all subgroups, with a biologically consistent gradient of response observed across the three genetic profiles.
Patients in Subgroup A showed the greatest clinical benefit (difference between BH-200 and placebo in HAM-D17 change from baseline at Week 8: -4.47; SE 1.58; p = 0.005), followed by Subgroup B (–2.85; SE 1.36; p = 0.037), and Subgroup C (–1.94; SE 1.35; p = 0.153).
Subgroup A, n = 89
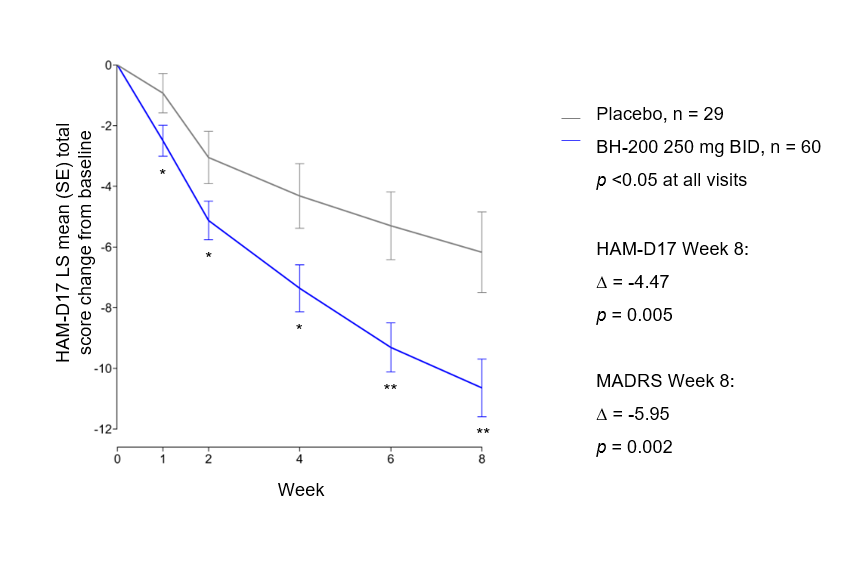
Patients in Subgroup A showed a rapid improvement in depression with separation from placebo on the HAM-D17 scale as early as Week 1 (p = 0.027) and maintained at every subsequent assessment. By Week 8, the mean difference versus placebo was -4.47 (SE 1.58; p = 0.005). On the Montgomery-Åsberg Depression Rating Scale (MADRS) change from baseline, the mean difference versus placebo in Subgroup A at Week 8 was -5.95 (SE 1.81, p = 0.002).
BH-200 was generally safe and well tolerated. The most common treatment-emergent adverse event was headache (reported in 8.9% of patients on BH-200). Three serious adverse events - allergic purpura, pneumonia, and acute stress - were reported, in two patients, all occurring in the placebo group. Asymptomatic and transient elevations in liver aminotransferase enzymes (AST or ALT > 3x ULN) were observed in 5.8% of patients in the BH-200 group; all cases resolved without clinical consequence. HMNC is currently exploring biomarker-based approaches to identify potential predictive indicators of liver enzyme elevations associated with BH-200 treatment.
Management Thoughts & Next Steps:
“The OLIVE trial results confirm earlier clinical trial data, validating vasopressin modulation as a novel and viable treatment approach in depression,” said Hans Eriksson, MD, PhD, MBA, Chief Medical Officer of HMNC. “Interestingly, the pre-defined patient Subgroup A showed a clinically robust response beginning at Week 1, highlighting the power of our genetic selection tool to identify those most likely to benefit. This marks a major step forward for precision psychiatry. We will now integrate the OLIVE data into our multi-omics platform to further enhance its predictive performance.”
Professor Dr. Dr. Dr. h.c. mult. Florian Holsboer, former director of the Max Planck Institute of Psychiatry, HMNC Brain Health co-founder and Head of the Scientific Advisory Board, commented, “The central role of vasopressin in the neurobiology of depression inspired the development of BH-200, a selective V1b receptor antagonist. In a large, controlled clinical trial, we were able to clearly demonstrate that a genetics-guided selection tool can identify the patient population that responds most favorably when this novel mechanism is targeted. What began as a bold hypothesis for precision psychiatry has now become a clinical reality.”
Maximilian Doebler, PhD, Chief Business Officer of HMNC Brain Health said, “We now have results from two large, well-conducted, randomized trials with more than 600 patients - one in the U.S., one in Europe - both showing clinically meaningful improvement in MDD. With OLIVE, we have taken a major step forward, identifying a substantial subset of patients who show early and strong responses. This is what precision psychiatry aims to achieve in clinical practice. These results give us strong confidence that the novel HPA-axis modulator (HPAM) mechanism of action, together with our genetic selection tool, can fundamentally change how we treat depression—by matching patients with the right therapy from the outset and moving beyond the limitations of trial-and-error prescribing.”
“The OLIVE trial results are important,” said Alan F. Schatzberg, MD, Professor of Psychiatry and Behavioral Sciences at Stanford University and Director of the Stanford Mood Disorders Center. “We’ve long understood the relevance of the HPA-axis in stress-related depression, but this is the first time we’ve seen clear improvement in MDD through vasopressin modulation. The clinical utility that is shown via the overall result, is also supported by early and robust response in an a priori defined subgroup (A), defined along genetic and HPA-axis variation. This could represent a paradigm shift toward more biologically informed treatment decisions in psychiatry.”
HMNC Brain Health will now initiate regulatory discussions for Phase 3 development of BH-200, leveraging its proprietary genetic selection tool to bring precision psychiatry into clinical practice. BH-200 could represent the first precision-guided antidepressant in real-world practice, changing how depression is treated at scale.
For more information on HMNC Brain Health or the OLIVE trial, please visit: www.hmnc-brainhealth.com.
ABOUT HMNC BRAIN HEALTH
HMNC Brain Health (HMNC Holding GmbH) is a precision psychiatry biopharmaceutical company developing personalized treatments for depression based on predictive genetic selection tools. The company’s pipeline includes three Phase 2 programs in Major Depressive Disorder (MDD): Nelivabon, with a vasopressin V1b receptor antagonist guided by a proprietary genetic selection tool; Cortibon, with a CRHR1 antagonist with a matching genetic selection tool; and KET01, a prolonged-release oral ketamine formulation designed for safe, at-home treatment.
Media contacts:
Anne Donohoe
+1 212-896-1265
Investors:
Maximilian Doebler
Chief Business Officer
HMNC Brain Health
maximilian.doebler@hmnc-brainhealth.com
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/3231ed22-64a4-4b27-9b4a-1eda77ceaf1f
https://www.globenewswire.com/NewsRoom/AttachmentNg/cc59a949-9ca9-48b7-a7fc-48b9349390c6
https://www.globenewswire.com/NewsRoom/AttachmentNg/c591833a-3e57-4e3b-9334-9769f92e43f7

More News
View More



Recent Quotes
View More
Quotes delayed at least 20 minutes.
By accessing this page, you agree to the Privacy Policy and Terms Of Service.