Clinical-state biopharmaceutical company Altimmune Inc. (NASDAQ: ALT) focuses on developing peptide-based therapeutics for obesity and chronic liver diseases, including non-alcoholic steatohepatitis (NASH). Their top candidate is a GLP-1/glucagon agonist called pemvidutide. It's a dual receptor agonist like Eli Lilly & Co. (NYSE: LLY) Mounjaro and Zepbound.
GLP-1 and Glucagon: Altimmune's Dual Target Approach
As a dual agonist, it could be more effective than Novo Nordisk A/S (NYSE: NVO) Wegovy or Ozempic for weight loss as they solely target the GLP-1 receptor, which is a hormone produced after eating that makes one feel full and lowers blood sugar. Glucagon increases blood sugar levels. Additionally, pemvidutide also targets NASH and metabolic dysfunction-associated steatohepatitis (MASH). Positive Phase 2 clinical data results surged shares 30% before pulling back down.
Altimmune operates in the medical sector and competes with other GLP-1 developers and providers like Viking Therapeutics Inc. (NASDAQ: VKTX), Pfizer Inc. (NYSE: PFE), and Structure Therapeutics Inc. (NASDAQ: GPCR).
Pemvidutide's Dual Action and the Role of Agonists
Pemvidutide is a peptide-based GLP-1/glucagon dual receptor agonist for treating obesity and MASH. GLP-1 suppresses appetite, and glucagon increases energy expenditure. Glucagon directly affects hepatic fat metabolism, which is believed to reduce liver fat levels and serum lipids. The FDA has fast-tracked pemvidutide for the treatment of MASH.
An agonist is a chemical substance that binds to a target receptor and activates it to produce a biological response. In other words, it mimics the hormone. Examples of agonists are morphine, an opioid agonist that binds to opioid receptors in the nervous system and brain, which leads to pain relief. Epinephrine is an agonist on adrenergic receptors, which causes increases in blood sugar and heart rate. Albuterol is a beta-2 adrenergic agonist, a receptor in the lungs that relaxes airways to help with breathing.
Altimmune's Phase 2 Clinical Trials: 48 Weeks of Pemvidutide, Diet and Exercise
The 48-week Phase 2 MOMENTUM Trial data were presented on June 23, 2024. The study had 391 participants with obesity and at least 1 co-morbidity but no diabetes. The subjects were randomized 1:1:1:1 to 1.2 mg, 1.8 mg, and 2.4 mg pemvidutide or placebo in conjunction with diet and exercise.
By week 48, subjects achieved an average weight loss of 10.3%, 11.2%, and 15.6% at the 1.2 mg, 1.8mg and 2.4 mg doses, respectively, and 2.2% for the placebo. Compared to 15% in 10 months with Ozempic. There were also robust reductions in serum lipids and improvements in blood pressure without imbalances in cardiac events, arrhythmias and clinically meaningful increases in heart rates. There were also significant decreases in heart disease risk factors like cholesterol.
Pemvidutide Versus Ozempic: Superior Lean Mass Preservation and Weight Loss
Full body composition MRIs of 50 subjects revealed that pemvidutide users showed the average lean mass loss was 21.9%, and 78.1% of the weight loss was body fat. This detail makes it a key factor versus Ozempic, as a 2021 study of a once-weekly 2.4 mg Semaglutide injection resulted in an average lean mass loss of 40%, and 60% of the weight loss was body fat. Additionally, average weight loss was 14.9% on week 68.
Altimmune CEO Vipin Garg commented, “We’re pleased with the data presented at ADA that highlight the impressive lean mass preservation achieved with pemvidutide, with only 21.9% of weight loss attributable to lean mass.”
Garg concluded, “The preservation of lean mass observed in this trial was better than reported historically with diet and exercise programs and greater than what has been publicly reported with other incretin weight loss drugs, where lean mass has accounted for as much as 40% of total weight loss… We believe that the level of muscle preservation observed in the Phase 2 trial further adds to the differentiation of pemvidutide in the treatment of obesity.”
Altimmune is a Speculative Lottery Ticket Bet
Investors should be aware that at this time, Altimmune has no FDA-approved drugs and hasn't yet generated significant revenues. Pemvidutide (ALT-801) is its sole pipeline drug, so it’s an all-in bet for the company. As of the close of the company's Q1 2024 on March 31, 2024, it had $181.1 million in cash and cash equivalents. Net loss was $24.4 million. The company is meeting with the FDA in Q3 2024 and expects to start Phase 3 clinical trials in Q1 2025.
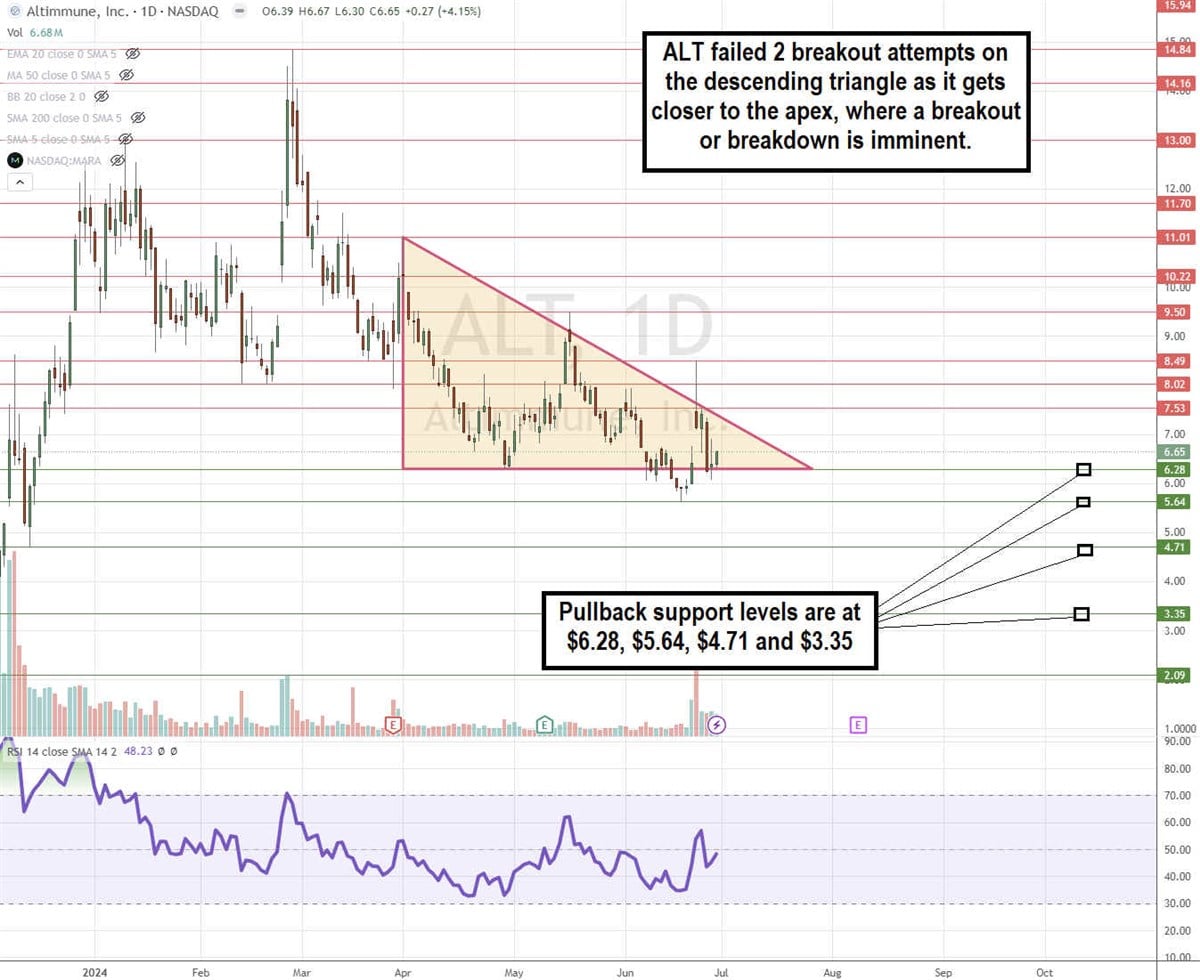
ALT Stock is in a Descending Triangle Pattern
Despite the favorable Phase 2 data, ALT is still in a daily descending triangle pattern. The breakout attempts to $9.50 on May 16, 2024, and $8.49 on June 24, 2024, still pulled the stock back down under the descending trendline. The flat-bottom trendline is at $6.28. The daily relative strength index is still coiled, attempting to bounce at the 50-band. Pullback support levels are at $6.28, $5.64, $4.71, and $3.35.
Altimmune analyst ratings and price targets are at MarketBeat.
