Pharmather Hldgs Ltd (OP: PHRRF )
0.1630
+0.0013
(+0.80%)
Streaming Delayed Price
Updated: 3:58 PM EST, Nov 14, 2024
Add to My Watchlist
All News about Pharmather Hldgs Ltd




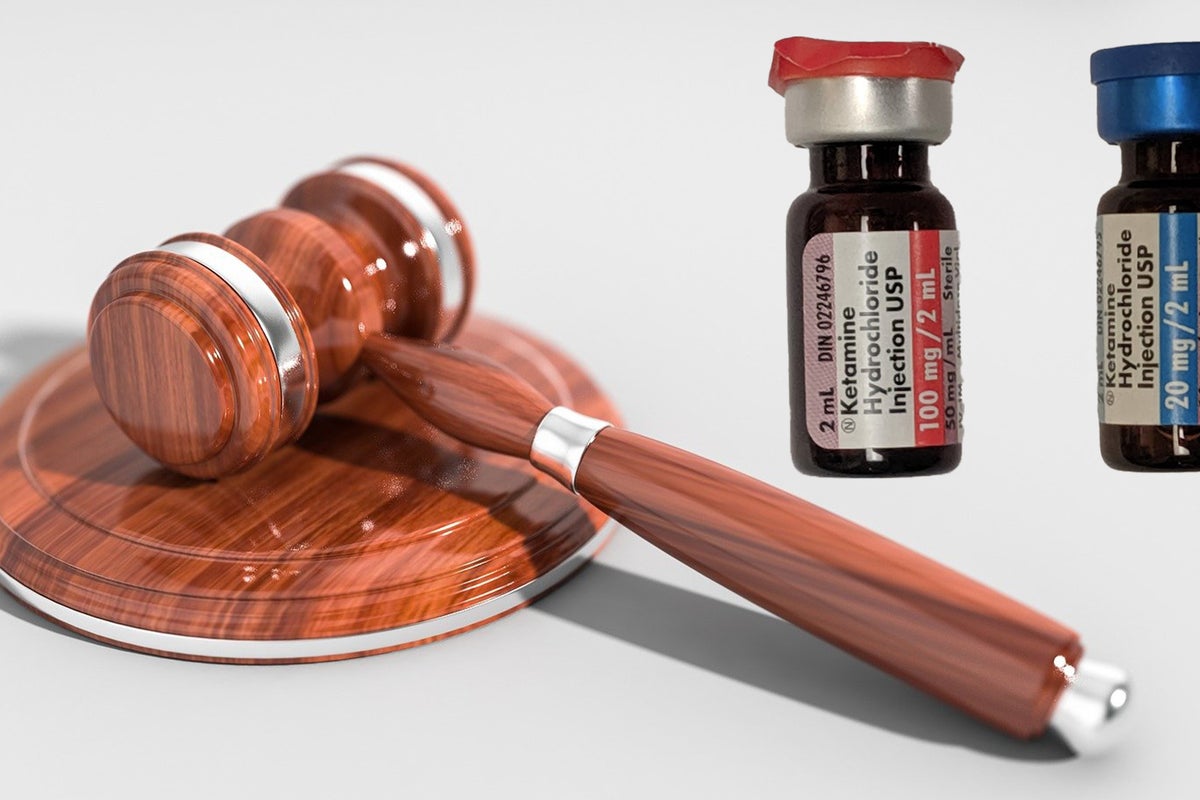
PharmaTher Announces Update on FDA New Drug Application for Ketamine
September 04, 2024
Via GlobeNewswire













Via Benzinga
Exposures
Product Safety



Psyched: Psilocybin Therapy For Depression, UK Rescheduling, US Policy Reform And More
September 11, 2023
Via Benzinga
Topics
Artificial Intelligence
Exposures
Artificial Intelligence
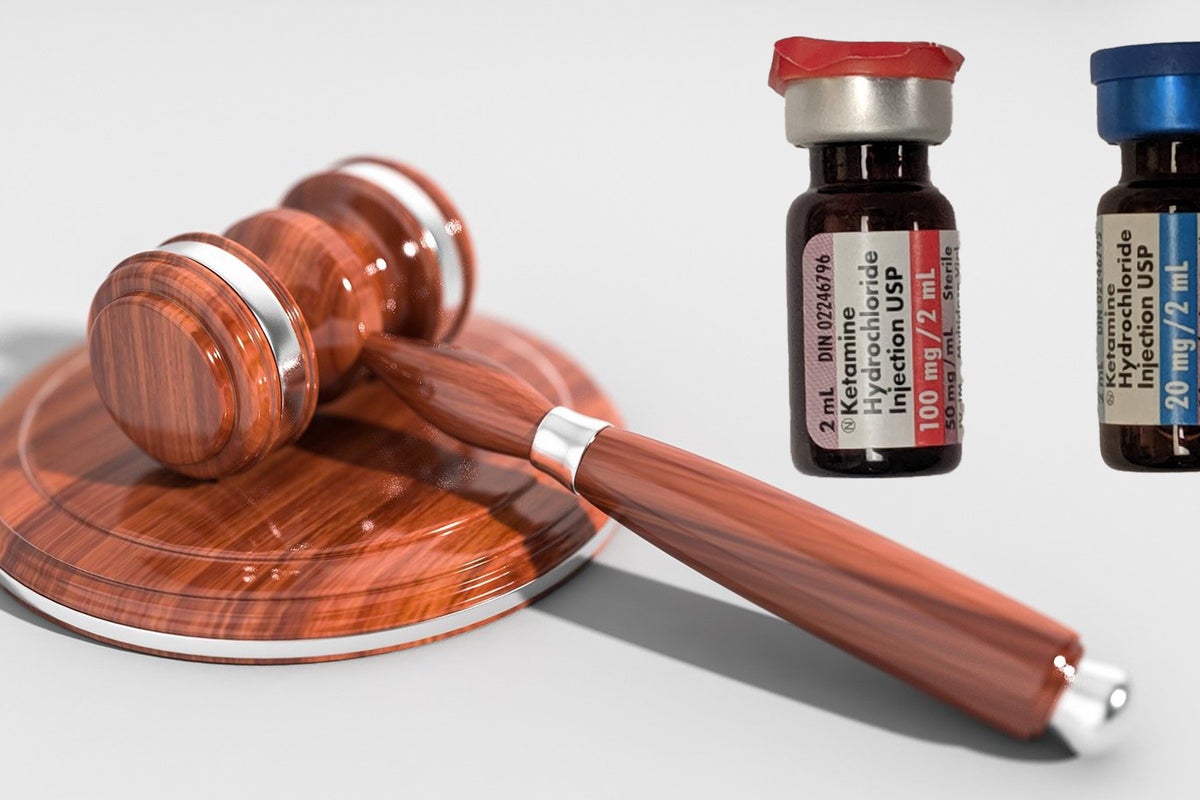
PharmaTher Aims To Mitigate Ketamine Shortage With New FDA Application
September 06, 2023
Via Benzinga
Exposures
Product Safety


Via Benzinga



Data & News supplied by www.cloudquote.io
Stock quotes supplied by Barchart
Quotes delayed at least 20 minutes.
By accessing this page, you agree to the following
Privacy Policy and Terms and Conditions.
Stock quotes supplied by Barchart
Quotes delayed at least 20 minutes.
By accessing this page, you agree to the following
Privacy Policy and Terms and Conditions.
