
On Dec. 8, 2023, Vertex Pharmaceuticals Inc. (NASDAQ: VRTX) announced the U.S. FDA approved its sickle-cell disease (SCD) treatment, Casgevy, for patients 12 years or older with recurrent vaso-occlusive crises (VOCs). Casgevy was co-developed with CRISPR Therapeutics AG (NASDAQ: CRSP). It utilizes CRISPR/Cas9 technology to perform genome editing, allowing scientists to alter, modify, add, or remove genetic material at specific locations in the genome.
The treatment has been 93.5% effective for treating SCD patients for VOCs. Vertex announced that the single treatment would be priced at $2.2 million per person. This revolutionary technology in the medical sector could be expanded to treat a universe of hereditary diseases in the future. The race is on in the gene-editing segment, with the likes of Intellia Therapeutics Inc. (NASDAQ: NTLA) and Beam Therapeutics Inc. (NASDAQ: BEAM) nipping at its heels.
What is CRISPR/Cas9 technology?
Every cell in the human body contains a copy of its genome. The human genome contains 24 distinct types of 46 DNA molecules requiring three billion pairs to be sequenced to gain complete information on a human's genome. Clustered Regularly Interspaces Short Palindromic Repeats (CRISPR) is a naturally occurring family of DNA sequences found in archaea and bacteria.
CRISPR is comprised of Cas9, a protein enzyme that can cut DNA, and a guide RNA that can identify the precise sequence of DNA to be edited. With CRISPR/Cas9, scientists first identify the DNA sequence that is causing a disease or malady and then create a specific guide RNA to target the sequence within the DNA.
The guide RNA is attached to the Cas9 and introduced into the diseased cells to locate and remove the targeted sequence. Scientists can edit the remaining genome by modifying, inserting or deleting new sequences. This is a cut-and-paste tool to edit the genome—the possibilities for treating and curing inherited diseases are monumental.
How Casgevy works
Casgevy is the first CRISP-based gene therapy to be approved for blood disorders. It’s an en vivo method that is approved for SCD and transfusion-dependent beta-thalassemia (TDT). Doctors take a sample of the patient’s blood stem cells and treat them in the lab with Casgevy.
Casgevy uses CRISPR/Cas9 to make specific changes to a specific gene called BCL11A, which suppresses the production of fetal hemoglobin after birth. The guide RNA is like a heat-seeking missile targeting the sequence within the BCL11A gene. It uses the Cas9 molecular scissors to cut out the specific sequence that suppresses the production of fetal hemoglobin. This enables BCL11A to continue producing fetal hemoglobin in the edited blood stem cells, which are then infused back into the patient’s bloodstream.
Benefits of fetal hemoglobin
Fetal hemoglobin is more efficient at transporting oxygen than adult hemoglobin. Increased fetal hemoglobin can improve oxygen delivery, reduce the sickling of red blood cells and decrease the need for blood transfusions. The infused stem cells travel to the bone marrow to start producing new healthy red blood cells with the increased fetal hemoglobin. Casgevy is intended to be a one-time treatment for SCD and TDT. Check out the sector heatmap on MarketBeat.
Casgevy promising results
The primary endpoint for Phase 2 and Phase 3 trials was to achieve no vaso-occlusive crises (VOCs) for at least one year in SCD patients. VOCs are the severe pain crises suffered from SCD. VOCS occurs from the production of hemoglobin S (HbS), which are sickle-shaped red blood cells that are rigid and clump together, blocking small blood vessels and causing severe pain that can lead to strokes and organ damage.
Casgevy had 29 out of 31 patients, or 93.5% of patients, achieved its primary endpoint and marked a significant reduction in painful episodes. Casgevy vastly increased fetal hemoglobin production, reaching average levels of around 30%. Casgevy is intended as a one-time treatment for SCD, but the company is exploring other hereditary diseases. Get AI-powered insights on MarketBeat.
Analysts downgrade
On Dec. 11, 2023, Cowen downgraded shares of CRISPR Therapeutics to Underperform from Market Perform with a $30 price target. Analysts don't feel it will be used broadly, and the stock is overvalued from a short squeeze. Oppenheimer analyst Jay Olson felt the FDA approval made history with the first-ever CRISPR-based approval in the United States, with more to expect in 2024. Casgevy also reviewed a priority review voucher (PRV), which paves the way for a quicker path to market. CRSP shares fell nearly 20% following the FDA approval.
CRISPR Therapeutics analyst ratings and price targets are at MarketBeat. CRISPR Therapeutics peers and competitor stocks can be found with the MarketBeat stock screener. CRSP shares have a 20.06% short interest.
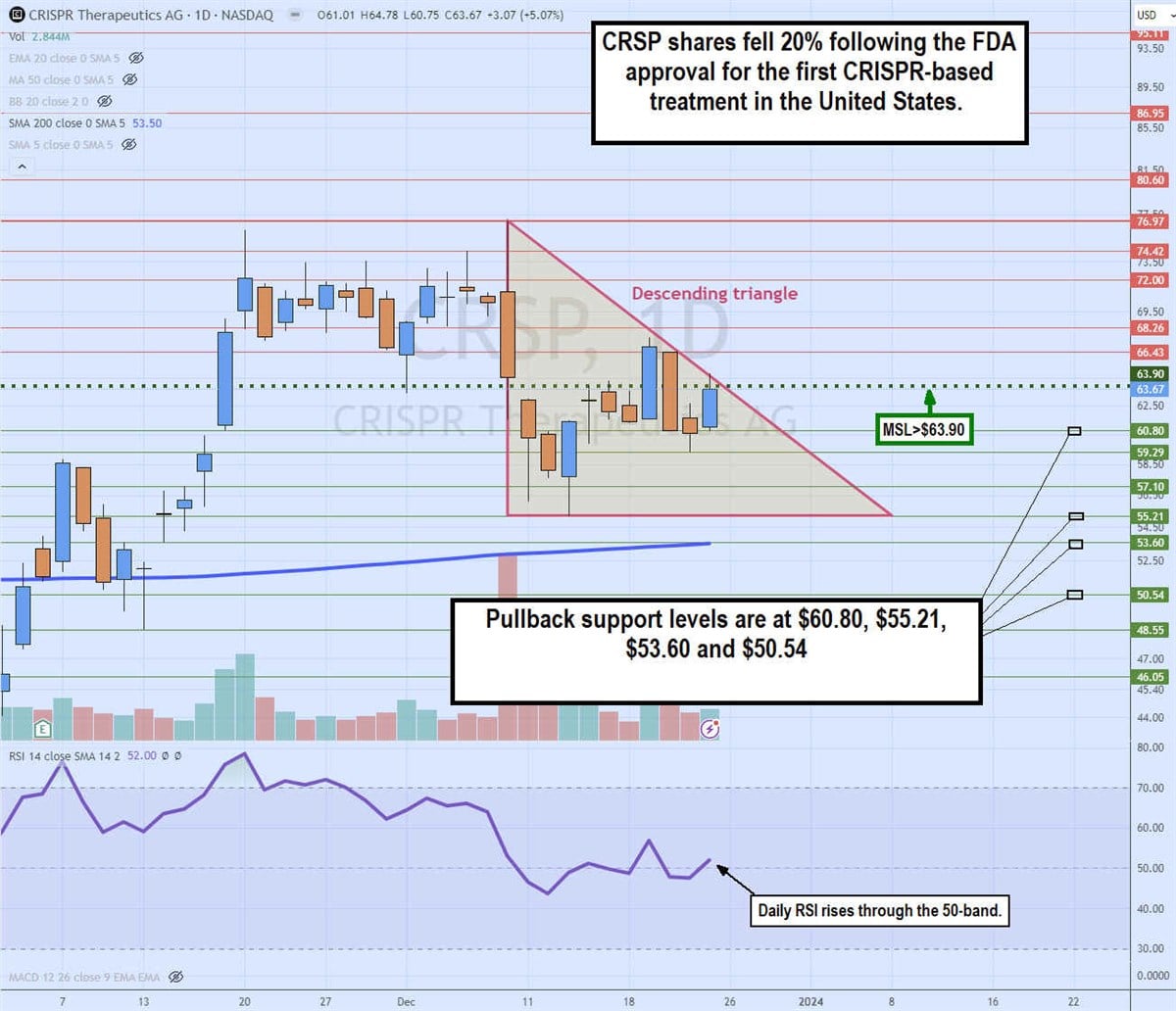
Daily descending triangle
The daily candlestick chart on CRSP illustrates a Descending triangle pattern. The descending trendline formed after peaking at $76.97 on Dec. 8, 2023, on the FDA approval announcement. A sell-the-new reaction caused shares to tumble over 20% to a low of $55.21 on Dec. 13, 2023. The daily market structure low (MSL) breakout staged a rally through the $63.90 trigger, but shares continued to make lower highs on the bounce attempts.
The descending triangle held resistance as the relative strength index (RSI) attempted to rise through the 50-band. Pullback support levels are at $60.80, $55.21, $53.60 and $53.60 daily 200-period moving average.
This resulted in a breakout through the upper trendline, squeezing back up to the $126.42 swing high and then gapping above, reaching $132.95. The very overbought RSI will eventually result in a pullback. Pullback support levels are at $118.63, $114.22, $108.55 and $104.33.
