Articles published by Lantern Pharma Inc.

Lantern Pharma Reports Second Quarter 2023 Financial Results and Operational Highlights
August 09, 2023
From Lantern Pharma Inc.
Via Business Wire
Tickers
LTRN

Lantern Pharma to Report Second Quarter 2023 Operating & Financial Results on August 9, 2023 at 4:30 p.m. ET
August 02, 2023
From Lantern Pharma Inc.
Via Business Wire
Tickers
LTRN



Lantern Pharma Receives FDA Clearance of IND Application for Drug Candidate LP-184 in Solid Tumors
June 12, 2023
From Lantern Pharma Inc.
Via Business Wire
Tickers
LTRN








Lantern Pharma Reports Fourth Quarter and Fiscal Year 2022 Financial Results and Operational Highlights
March 20, 2023
From Lantern Pharma Inc.
Via Business Wire
Tickers
LTRN

Lantern Pharma to Present at the American Association for Cancer Research (AACR) Annual Meeting
March 15, 2023
From Lantern Pharma Inc.
Via Business Wire
Tickers
LTRN



Lantern Pharma Announces New Data and Development Focus for LP-100 with PARP Inhibitors
March 09, 2023
From Lantern Pharma Inc.
Via Business Wire
Tickers
LTRN
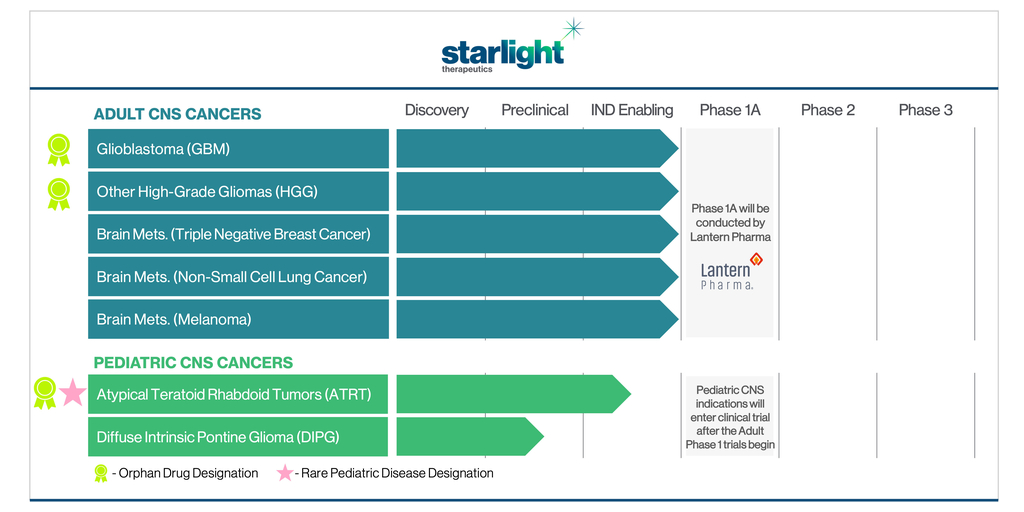





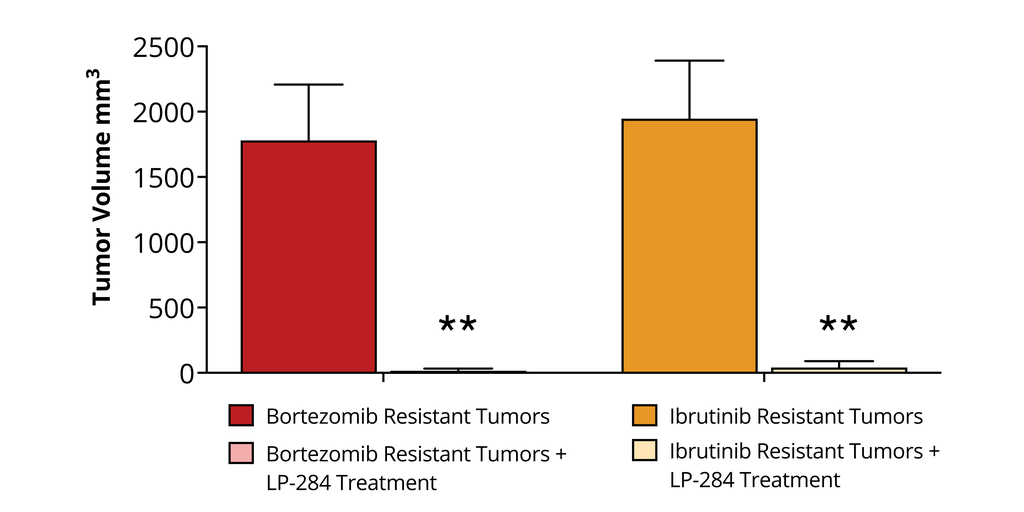
FDA Grants Lantern Pharma Orphan Drug Designation for Drug Candidate LP-284 in Mantle Cell Lymphoma
January 05, 2023
From Lantern Pharma Inc.
Via Business Wire
Tickers
LTRN



Lantern Pharma Reports Third Quarter 2022 Financial Results and Operational Highlights
November 07, 2022
From Lantern Pharma Inc.
Via Business Wire
Tickers
LTRN


Lantern Pharma to Report Third Quarter 2022 Operating & Financial Results on November 7, 2022 at 4:30 p.m. ET
October 31, 2022
From Lantern Pharma Inc.
Via Business Wire
Tickers
LTRN

Lantern Pharma to Present New Preclinical Data at Three Upcoming Scientific Conferences
October 25, 2022
From Lantern Pharma Inc.
Via Business Wire
Tickers
LTRN
Data & News supplied by www.cloudquote.io
Stock quotes supplied by Barchart
Quotes delayed at least 20 minutes.
By accessing this page, you agree to the following
Privacy Policy and Terms and Conditions.
Stock quotes supplied by Barchart
Quotes delayed at least 20 minutes.
By accessing this page, you agree to the following
Privacy Policy and Terms and Conditions.