Movano Inc. Provides Business Update and Reports Second Quarter 2021 Financial Results
Conference call begins at 2:00 p.m. Pacific time today
PLEASANTON, CA / ACCESSWIRE / August 12, 2021 / Today, Movano Inc. (NASDAQ: MOVE), a health technology company designing devices that empower individuals to optimize their health in order to help prevent and better manage chronic diseases, reported financial results for the three months ending June 30, 2021 and provided a business update. Highlights from the second quarter and recent weeks include the following:
- During the second quarter 2021, the Company received approval from the Institutional Review Board (IRB) to conduct blood pressure studies with up to 200 participants at the Movano Clinical Lab, located in its corporate office space. In late-June 2021, the Company used its noninvasive, iPhone-sized prototype to collect pulse pressure waveform data from 45 external volunteers with varying gender, age, weight, ethnicity and blood pressure. During each session, participants wore Movano's prototype along with a hospital-grade FDA-cleared vital signs monitor as the control. The Company expects to use this data to further evaluate key modeling factors and continue to improve upon blood pressure algorithm development. The study marked an important milestone for Movano as it confirmed the Company's ability to efficiently enroll subjects and conduct testing in its own space, enabling Movano's Clinical team to have more opportunities to collect data as needed in the future.
- Movano announced today that its wearable device prototype could be operational and out of the development stage by the end of the third quarter or early fourth quarter 2021 and ready for an upcoming clinical study shortly thereafter, both of which would be significant milestones and steps forward in productizing the Company's technology. With this smaller, wrist-worn device, Movano will be transitioning from an iPhone-sized, tethered prototype to the first iteration of its completely wireless, battery powered wearable device. The new device is expected to advance testing capabilities, improve the quality and quantity of data collected, and enable Movano's Clinical team to conduct longer and more complex studies. Movano plans to conduct a blood pressure study in the fourth quarter, under an approved IRB protocol, using the new wearable device.
- During the second quarter 2021, Movano continued to progress with the miniaturization of its technology toward a single-chip solution. Once developed, the single-chip solution will package all of the Company's integrated circuits (ICs) into one IC, providing more flexibility in clinical testing and creating the foundation for the development of initial commercial products.
"The healthcare industry is transitioning from a practice of treating the sick to a consumer-driven health market focused on preventative care and longevity. Most recently, the pandemic has affected everyone - those with chronic conditions or not - and it has accelerated the desire for a connected, digitally-enabled health and wellness platform, predicated on devices that provide medical-grade diagnostics in addition to lifestyle fitness statistics," said Dr. John Mastrototaro, Movano's CEO. "We plan to meet the new demands of consumers by developing products that are at the intersection of the medical and consumer device market with technology that is simple, smart and personalized. Our noninvasive medical devices will come in multiple form factors and empower individuals with more frequent and digestible data, whether they have or are at risk of diabetes, hypertension, or are committed to a healthy lifestyle. As we continue to build our end-to-end system and miniaturize our technology, we are focused on creating devices that make it easy for consumers to be more proactive about their health and support better health choices in order to help mitigate, delay or avoid the effects of chronic disease."
Second Quarter 2021 Financial Results
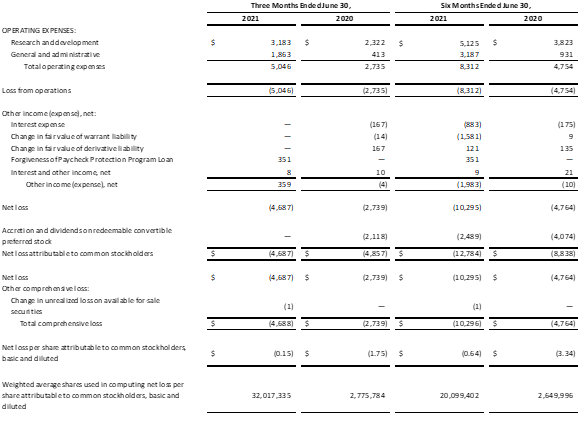
- Movano reported a net loss attributable to common stockholders of $4.7 million, or a loss of $0.15 per basic and diluted share, in the second quarter of 2021, compared with a net loss attributable to common stockholders of $4.9 million, or a loss of $1.75 per basic and diluted share, in the second quarter of 2020.
- The Company reported an operating loss of $5.0 million in the second quarter of 2021 compared to an operating loss of $2.7 million in the second quarter of 2020.
- Movano is a development stage company and the majority of our business activities to date and our planned future activities will be devoted to research and development. As such, the Company did not generate revenue in either the second quarter of 2021 or the second quarter of 2020.
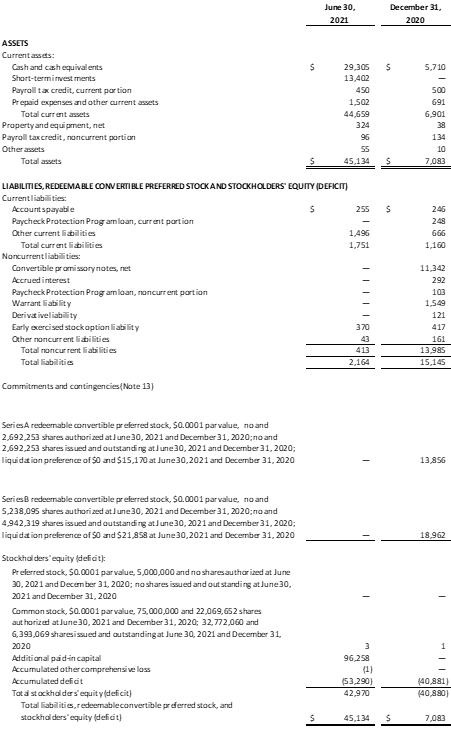
- The Company had $42.7 million in cash, cash equivalents and short-term investments as of June 30, 2021, compared to $5.7 million in cash, cash equivalents and short-term investments, as of December 31, 2020.
- The total number of shares outstanding was 32.8 million as of June 30, 2021.
Conference Call and Webcast
Management will host a conference call and live audio webcast to discuss these results and provide a business update today at 2:00 p.m. PDT (5:00 p.m. EDT).
The live webcast can be accessed on the investors section of Movano's website at https://ir.movano.com. The conference call can be accessed by dialing 1-877-407-0989 (domestic) or 1-201-389-0921 (international) and refer to confirmation number 13721098. Attendees can also use the Call Me link, in which they will be dialed in to the conference call instantly on the number provided with no hold time. An archived webcast will be available on Movano's website approximately one hour after the completion of the event and for two years thereafter.
To learn more about Movano Inc., please visit www.movano.com
About Movano Inc.
Founded in 2018, Movano Inc. (NASDAQ: MOVE), is a health-focused technology company creating simple, smart and personalized devices designed to help individuals on their health journey optimize for good health today and prevent and manage chronic diseases in the future. Movano's technology is being developed to provide vital health information, including glucose and blood pressure data, in a variety of form factors to meet individual style needs and give users actionable feedback in order to improve their quality of life.
Forward Looking Statements
This press release contains forward-looking statements concerning our expectations, anticipations, intentions, beliefs or strategies regarding the future. These forward-looking statements are based on assumptions that we have made as of the date hereof and are subject to known and unknown risks and uncertainties that could cause actual results, conditions and events to differ materially from those anticipated. Therefore, you should not place undue reliance on forward-looking statements. Examples of forward-looking statements include, among others, statements we make regarding expected future operating results; product development, clinical trial and regulatory initiatives; our strategies, positioning and expectations for future events or performance. Important factors that could cause actual results to differ materially from those in the forward-looking statements are set forth in our registration statement on Form S-1, as amended, and any subsequent Quarterly Reports on Form 10-Q, and in our other reports filed with the Securities and Exchange Commission, including under the caption "Risk Factors." Any forward-looking statement in this release speaks only as of the date of this release. We undertake no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise.
Investor Relations Contact:
J. Cogan
IR@movano.com
Movano Inc.
Condensed Consolidated Balance Sheets
(in thousands, except share and per share data)
(Unaudited)
Movano Inc.
Condensed Consolidated Statements of Operations and Comprehensive Loss
(in thousands, except share and per share data)
(Unaudited)
SOURCE: Movano Inc.
View source version on accesswire.com:
https://www.accesswire.com/659388/Movano-Inc-Provides-Business-Update-and-Reports-Second-Quarter-2021-Financial-Results
More News
View More




Recent Quotes
View More
Quotes delayed at least 20 minutes.
By accessing this page, you agree to the Privacy Policy and Terms Of Service.