NAMSA Transforms Integrated MedTech Commercialization Solution: the NAMSA APEX Program™
Strategic Outsourcing Solution Provides Predictable Development Outcomes for Successful MedTech Regulatory Approval and Market Adoption
NAMSA, a world-leading MedTech Contract Research Organization (CRO) offering global end-to-end development services, announced today the transformation of its integrated MedTech commercialization solution: the NAMSA APEX Program™. The relaunch and transformation of the Program reflects NAMSA’s continued focus on strategic outsourcing solutions to address development challenges encountered by MedTech Sponsors, resultant from current market conditions and demands.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20221117005317/en/
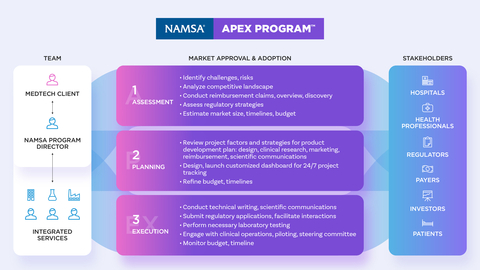
The NAMSA APEX Program: Strategic Outsourcing Solution for Predictable MedTech Development Outcomes (Graphic: Business Wire)
NAMSA’s strategic outsourcing solution, the NAMSA APEX Program (Assess, Plan, Execute), is introduced at a time when the MedTech industry is experiencing changing regulatory requirements and expanding clinical evidence demands. Sponsors are facing longer development timelines, putting them at risk for increased costs and lagged speed-to-market—often the key drivers of MedTech success. Helping Sponsors plan for these challenges is the NAMSA APEX Program: an approach that delivers integrated services and tools that provide predictable planning, phase overlap and immediate access to top Subject Matter Experts in Clients’ therapeutic, geographic and regulatory focus areas. This ultimately allows Sponsors to proactively mitigate risk, preserve capital and create stakeholder alignment to accelerate commercialization.
Commenting on NAMSA’s transformed strategic outsourcing solution is Tim Mitchell, NAMSA Vice President of Strategic Programs, “In today's value-based environment, the commercial challenges are more numerous than ever as regulatory approval does not guarantee market adoption. This has placed increased demand on MedTech Sponsors to find trusted outsourcing partners to accelerate efficient clinical development of innovative medical products.” Mitchell continued, “NAMSA’s APEX Program is one approach to help ensure an efficient path to commercial success. As a result of our work with more than 100 APEX Program Clients, we understand how to set the stage for efficient product approval and market adoption through our established regulatory partnerships and utilization of only the most proven development strategies.”
MedTech developers wishing to learn more about the NAMSA APEX Program and other strategic outsourcing solutions are invited to sign-up for a complimentary consultation via the company’s website at: www.namsa.com/services/apex-program/.
ABOUT NAMSA
Helping medical device Sponsors improve healthcare since 1967, NAMSA is the world’s leading MedTech Contract Research Organization (CRO) offering global end-to-end development services. Driven by its global regulatory expertise and in-depth therapeutic knowledge, NAMSA is dedicated to accelerating medical device product development, offering only the most proven solutions to move clients’ products through the development lifecycle efficiently and cost-effectively. From medical device testing; regulatory, reimbursement and quality consulting; and clinical research services, NAMSA is the industry’s premier, trusted partner for successful development and commercialization outcomes. Web: namsa.com
View source version on businesswire.com: https://www.businesswire.com/news/home/20221117005317/en/
Contacts
NAMSA Media Contact
Leah Davidson, MA, MBA
Sr. Manager, Global Marketing Communications
Email: ldavidson@namsa.com
More News
View More



Recent Quotes
View MoreQuotes delayed at least 20 minutes.
By accessing this page, you agree to the Privacy Policy and Terms Of Service.