Preliminary Phase 1b/2 Data for REC-4881 in Familial Adenomatous Polyposis (FAP) Demonstrates Reduced Polyp BurdenMay 04, 2025 at 12:45 PM EDT
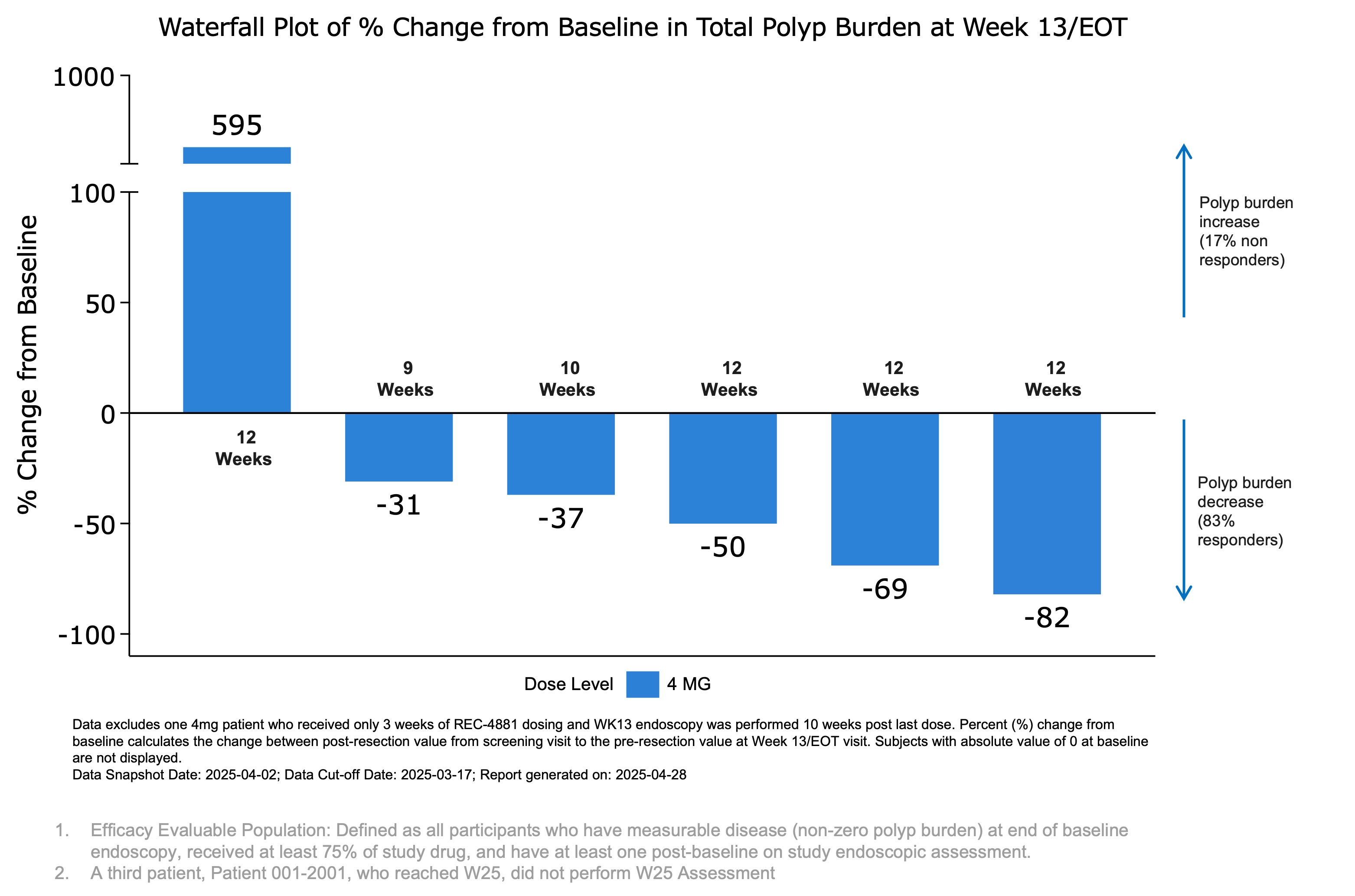
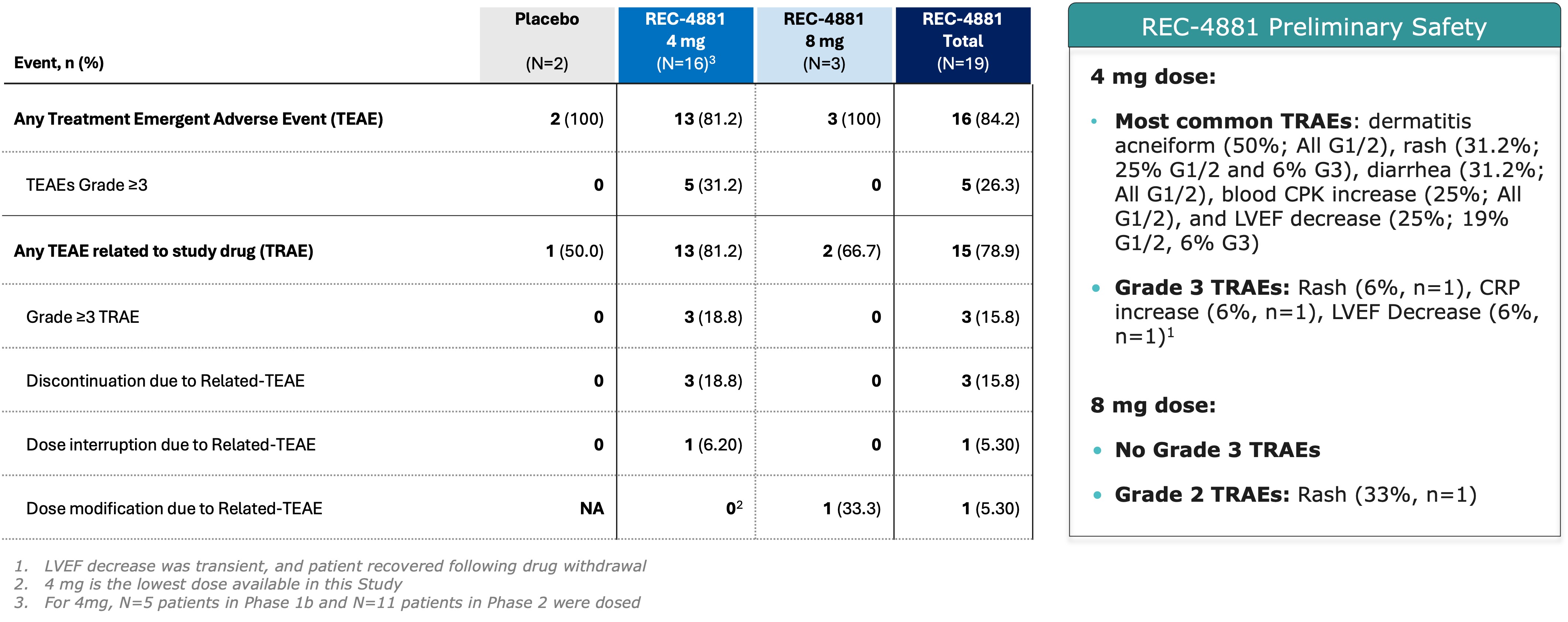
SALT LAKE CITY, May 04, 2025 (GLOBE NEWSWIRE) -- Recursion (Nasdaq: RXRX), a clinical-stage TechBio company decoding biology to radically improve lives, today announced preliminary safety and efficacy results from its ongoing Phase 1b/2 TUPELO trial of REC-4881, an investigational, allosteric MEK1/2 inhibitor in development for Familial Adenomatous Polyposis (FAP). These data were presented in a late-breaking oral presentation at Digestive Disease Week (DDW) 2025 in San Diego, California. FAP is a rare, inherited disorder caused by mutations in the APC gene, leading to the growth of hundreds to thousands of gastrointestinal polyps and a near 100% lifetime risk of colorectal cancer if left untreated. Despite this significant burden, there are currently no FDA-approved treatments. FAP affects an estimated 50,000 individuals across the US and EU5 (France, Germany, Italy, Spain, and the UK). REC-4881 has received Fast Track and Orphan Drug designations from the U.S. FDA, as well as Orphan Drug designation from the European Commission. As of the March 17, 2025 data cutoff in the open-label Phase 2 portion of the TUPELO trial, treatment with REC-4881 (4 mg QD) led to a preliminary median 43% reduction to date in polyp burden at the Week 13 assessment among six efficacy-evaluable patients. Other investigational agents in separate studies have reported 20-30% polyp burden reduction in 6 months1. Five of the six patients (83%) experienced reductions in polyp burden ranging from 31% to 82%, while one patient (17%) showed a substantial increase of 595% from baseline. Additionally, three of six patients (50%) achieved a ≥1-point reduction in Spigelman stage; one patient had no change, one progressed from Stage II to IV, and one had no baseline stage but was classified as Stage II at Week 13. Across the combined Phase 1b and ongoing Phase 2 cohorts (n=19 safety-evaluable patients), REC-4881 demonstrated an early safety profile generally consistent with that of prior MEK1/2 inhibitors. Seventy-nine percent (15 of 19) of patients experienced at least one treatment-related adverse event (TRAE), the majority of which were Grade 1 or 2 in severity. Grade 3 TRAEs occurred in 16% of patients, with no Grade ≥4 events reported to date. In Phase 1b, the most frequent TRAEs (≥20%) were acneiform rash, diarrhea, and decreased left ventricular ejection fraction (LVEF), while in Phase 2, the most common events were acneiform rash, blood creatine phosphokinase (CPK) increase, and diarrhea. Overall, treatment modifications were infrequent, with 3 of 19 patients discontinuing treatment, 1 patient each requiring dose interruption or modification. "For patients with FAP, who face increased risk of colorectal and small bowel cancer and a lifetime of invasive interventions, the preliminary polyp burden reduction at a median of >43% seen with REC-4881 1in just three months of treatment is highly encouraging," said Dr. Jewel Samadder, Gastroenterologist at Mayo Clinic and Principal Investigator of TUPELO, "These early results offer a much-needed glimpse of hope for this underserved population." "For patients with FAP, who currently lack FDA-approved treatment options, Recursion's AI-powered Recursion OS platform identified a promising approach through MEK 1/2 inhibition," said Najat Khan, PhD, Chief R&D Officer and Chief Commercial Officer at Recursion. "By analyzing cellular models of APC gene loss, we uncovered a potential first-in-disease treatment and are excited to share our preliminary findings." About the TUPELO Trial Design (REC-4881) The Phase 1b portion was a randomized, double-blind, placebo-controlled safety run-in designed to assess the safety, tolerability, and PK of REC-4881 at 4 mg QD for 14 days in FAP patients aged 18 years and older. A total of five patients received REC-4881, and two received placebo. Following the safety review, the eligibility criteria were amended to enroll only patients aged ≥55 years in Phase 2, in an effort to reduce treatment-related adverse events (TRAEs) consistent with MEK1/2 inhibition. In the ongoing Phase 2 portion, participants must have a confirmed germline APC mutation and be ≥55 years old. Efficacy is assessed via upper and lower endoscopy at baseline, Week 13 (W13, on-treatment), and Week 25 (W25, off-treatment). The primary efficacy endpoint is percent change from baseline in polyp burden. The Efficacy Evaluable Population includes patients who had measurable disease at baseline, received ≥75% of the study drug, and completed at least one post-baseline endoscopic assessment. Disease staging is evaluated using the Spigelman system for the upper gastrointestinal tract and the InSiGHT classification for the lower GI tract. Efficacy At Week 13, three of six patients achieved a ≥1-point reduction in Spigelman stage, a validated measure of upper gastrointestinal disease severity; one patient had no change, one progressed from Stage II to IV, and one had no baseline stage but was classified as Stage II at Week 13. At the time of data cutoff, three additional patients had been enrolled in the 8 mg QD dose cohort. All three were found to have no measurable disease (zero polyp burden) at baseline endoscopy and were therefore not considered efficacy evaluable.
Figure 1: Waterfall plot showing percent change from baseline in total polyp burden at Week 13 (end of treatment) for efficacy-evaluable patients receiving REC-4881 (4 mg QD). Safety Grade 3 TRAEs included rash, increased C-reactive protein (CRP), and decreased LVEF—each occurring in one patient (6%), all within the 4 mg dose cohort. A single patient in the Phase 1b cohort experienced a Grade 3 LVEF decrease, which was transient and resolved following dose interruption. No patients in Phase 1b discontinued treatment due to a TRAE. Three patients in Phase 2 discontinued treatment due to TRAEs. A full summary of adverse events is provided in Figure 2.
Figure 2. Summary of Adverse Events Across Phase 1b and Phase 2 of the TUPELO Trial Next Steps About REC-4881 About Recursion Forward-Looking Statements Media Contact Investor Contact Photos accompanying this announcement are available at https://www.globenewswire.com/NewsRoom/AttachmentNg/b64bdf18-73b8-4f07-b38d-a96478162b1b 1 Steinberg et al 2000 NEJM and Burke et al 2024 Gastroenterology 
More NewsView More
Up Over 20% in 2025, These 3 Stocks Are Boosting Buyback Capacity ↗
December 01, 2025
Via MarketBeat

Congress Beat the Market Again—Here Are the 3 Stocks They Bought ↗
December 01, 2025
Via MarketBeat

Go on a Shopping Spree With 3 Top Retail ETFs ↗
December 01, 2025

3 Fresh Dividend Hikes That Might Be Telling You Something ↗
December 01, 2025

Recent QuotesView More
Stock Quote API & Stock News API supplied by www.cloudquote.io
Quotes delayed at least 20 minutes. By accessing this page, you agree to the Privacy Policy and Terms Of Service.
© 2025 FinancialContent. All rights reserved.
|