Articles published by Edgewise Therapeutics



Edgewise Therapeutics Strengthens Leadership Team to Support Late-Stage Clinical Development
January 22, 2025
Via Business Wire
Tickers
EWTX

Via Business Wire
Tickers
EWTX

Edgewise Therapeutics to Present at the 43rd Annual J.P. Morgan Healthcare Conference on January 13, 2025
January 07, 2025
Via Business Wire
Tickers
EWTX


Edgewise Provides Statement Regarding Company’s Relationship with Dr. Han Phan at Rare Disease Research
December 05, 2024
Via Business Wire
Tickers
EWTX

Edgewise Therapeutics to Present at the Piper Sandler 36th Annual Healthcare Conference on December 3, 2024
November 26, 2024
Via Business Wire
Tickers
EWTX

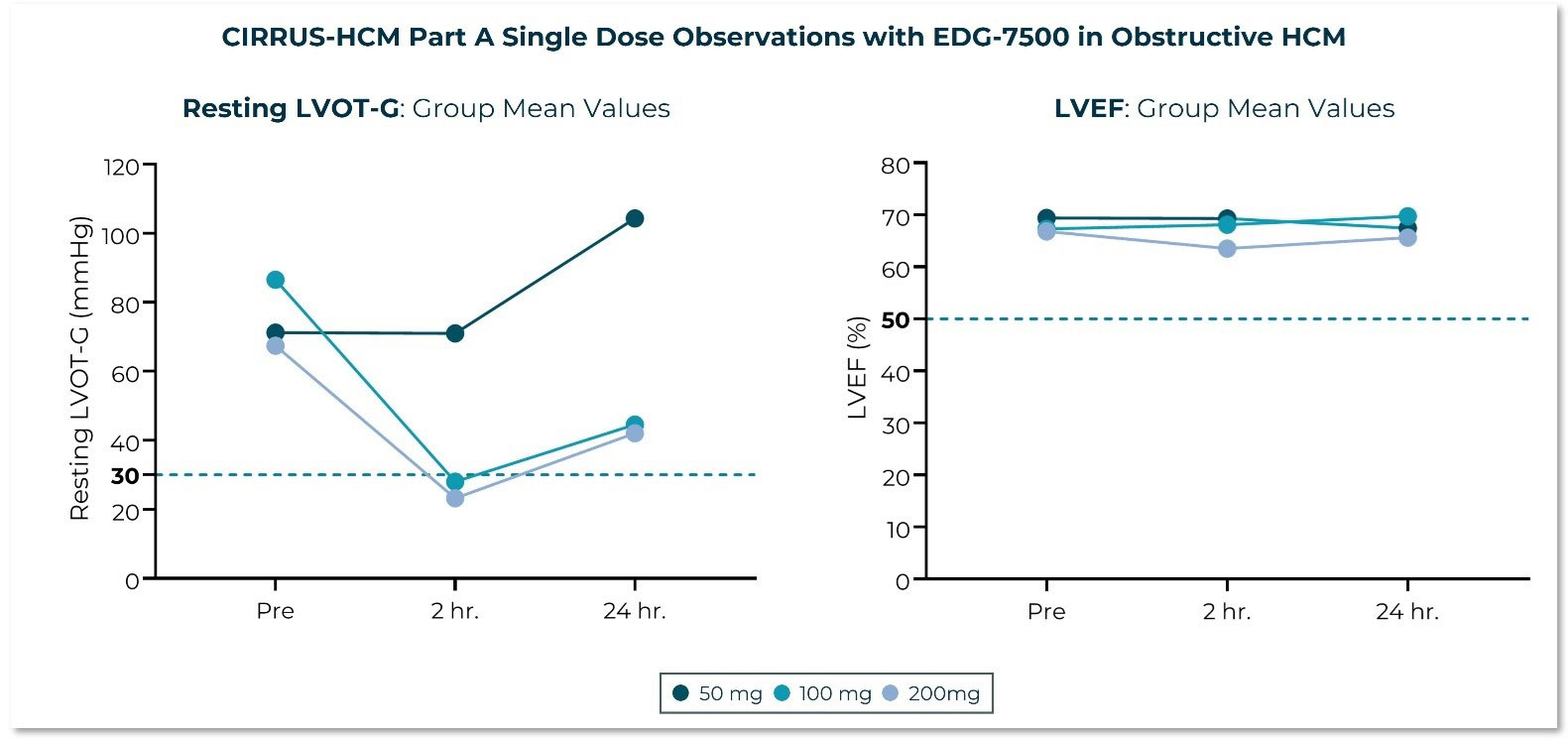
Edgewise Therapeutics Reports Third Quarter 2024 Financial Results and Recent Business Highlights
November 07, 2024
Via Business Wire
Tickers
EWTX


Via Business Wire
Tickers
EWTX

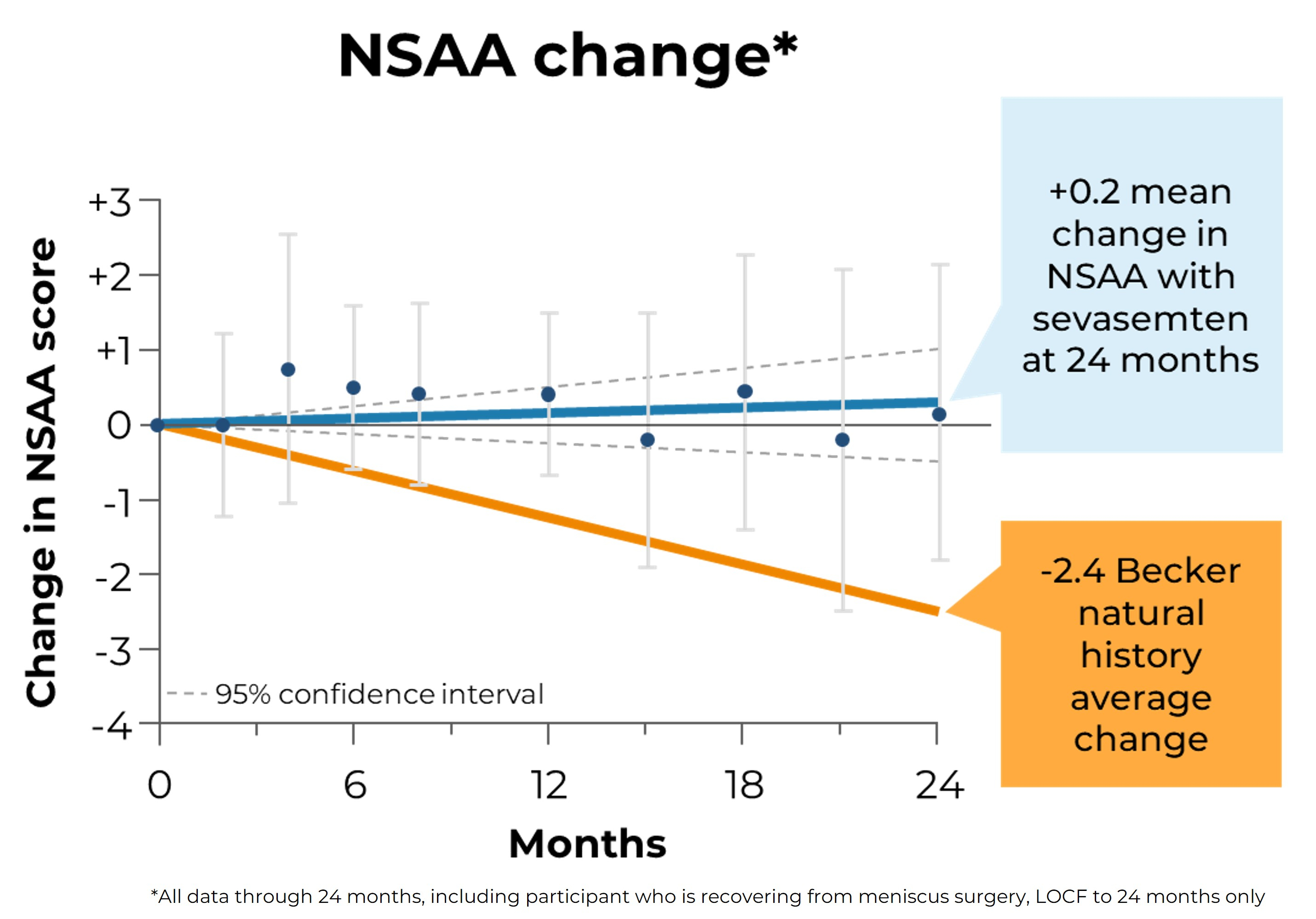
Edgewise Therapeutics Reports Second Quarter 2024 Financial Results and Recent Business Highlights
August 08, 2024
Via Business Wire
Tickers
EWTX



Via Business Wire
Tickers
EWTX









Edgewise Therapeutics Announces Pricing of $240 Million Underwritten Offering of Common Stock
January 19, 2024
Via Business Wire
Tickers
EWTX


Edgewise Therapeutics to Present at the 42nd Annual J.P. Morgan Healthcare Conference on January 9, 2024
December 19, 2023
Via Business Wire
Tickers
EWTX

Stock Quote API & Stock News API supplied by www.cloudquote.io
Quotes delayed at least 20 minutes.
By accessing this page, you agree to the Privacy Policy and Terms Of Service.
Quotes delayed at least 20 minutes.
By accessing this page, you agree to the Privacy Policy and Terms Of Service.
© 2025 FinancialContent. All rights reserved.
