Articles published by Clovis Oncology, Inc.

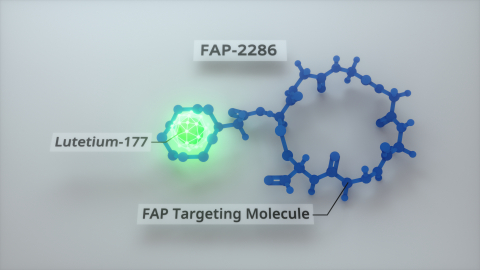
Clovis Oncology Files for Chapter 11 Protection and Enters into Agreement to Sell FAP-2286
December 12, 2022
Via Business Wire
Tickers
CLVS

Clovis Oncology and Isotopia Announce Lutetium-177 Clinical Supply Agreement
September 21, 2022
Via Business Wire
Tickers
CLVS




Via Business Wire
Tickers
CLVS


Via Business Wire
Tickers
CLVS







Via Business Wire
Tickers
CLVS

Clovis Oncology Announces 2021 Operating Results and Anticipated 2022 Development Milestones
February 23, 2022
Via Business Wire
Tickers
CLVS


Clovis Oncology Announces Preliminary Financial Results for the Fourth Quarter and Full Year 2021
January 10, 2022
Via Business Wire
Tickers
CLVS

Clovis Oncology Highlights FAP-2286 Preclinical Data Presented at the Targeted Radiopharmaceuticals Summit
December 09, 2021
Via Business Wire
Tickers
CLVS

Clovis Oncology Announces Third Quarter 2021 Operating Results
November 03, 2021
Via Business Wire
Tickers
CLVS


Via Business Wire
Tickers
CLVS


Clovis Oncology Retires Remaining 2021 Notes and Raises Additional Capital through its ATM Equity Offering Program
September 20, 2021
Via Business Wire
Tickers
CLVS

Via Business Wire
Tickers
CLVS

Clovis Oncology Announces Second Quarter 2021 Operating Results
August 04, 2021
Via Business Wire
Tickers
CLVS


Via Business Wire
Tickers
CLVS


Stock Quote API & Stock News API supplied by www.cloudquote.io
Quotes delayed at least 20 minutes.
By accessing this page, you agree to the following
Privacy Policy and Terms Of Service.
Quotes delayed at least 20 minutes.
By accessing this page, you agree to the following
Privacy Policy and Terms Of Service.
|
|
|