- The Phase 2 HARMONIC™ study is designed for never smokers with lung adenocarcinoma with the objective to overcome driver mutation(s) – which are more common in never smokers – that lead to tyrosine kinase inhibitor (TKI) treatment resistance, and to enhance patient outcomes when LP-300 is used with the standard-of-care chemotherapy doublet.
- In the Phase 2 lead-in cohort of 7 patients, 6 patients experienced clinical benefit from the combination of LP-300 and chemotherapy while 1 patient experienced progressive disease.
- Of the 6 patients experiencing clinical benefit – 3 patients showed partial responses with an average tumor size reduction of 51% and 3 patients have stable disease with an average tumor size reduction of 13%.
- The clinical benefit rate and disease control rate is 86% for this group with an objective response rate (ORR) of 43%.
- A preliminary analysis of data in the safety lead-in part of the trial indicates no additional safety concerns with no observed dose limiting toxicities (DLTs) and no reported treatment-related serious adverse events (SAEs).
- Encouraging preliminary efficacy results were observed regardless of prior tyrosine kinase inhibitor (TKI) treatment(s), demographics, and metastatic disease sites.
- In the initial set of patients, those having low to intermediate TMB (tumor mutation burden) were found to be responsive to LP-300 + chemotherapy.
- There are no currently approved therapies or targeted agents specifically approved for use in NSCLC among never-smokers, which is a growing global class of patients.
Lantern Pharma (NASDAQ: LTRN), an artificial intelligence (AI) company dedicated to developing cancer therapies and transforming the cost, pace, and timeline of oncology drug discovery and development, today announced positive, preliminary results from the initial patient group in the ongoing HARMONIC™ phase 2 clinical trial. HARMONIC™ is evaluating Lantern Pharma’s investigational new drug candidate, LP-300, in combination with pemetrexed and carboplatin in never smokers1 with advanced non-small cell lung cancer (NSCLC) who have progressed after receiving treatment with a tyrosine kinase inhibitor (TKI). LP-300 was advanced in part with Lantern’s AI platform, RADR®, to aid in the validation of mechanisms and uncover insights in targeted patient populations. RADR® is Lantern’s AI platform for cancer therapy discovery, development and rescue with over 100 billion data points and aiding in the development of both Lantern’s portfolio and development initiatives with Lantern’s collaborators.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240805396240/en/
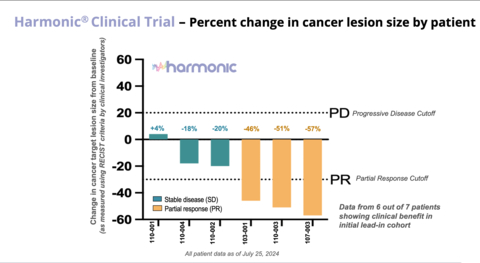
Harmonic® Clinical Trial - Percent change in cancer lesion size by patient (Graphic: Business Wire)
Summary of Harmonic™ Clinical Results
For the initial safety lead-in portion of the Phase 2 study, which involved 7 patients, all patients received the LP-300 drug candidate with pemetrexed and carboplatin intravenously2. Preliminary results at the completion of the 7-patient lead-in part of the Harmonic™ study demonstrated predictable safety profiles that are consistent with the chemotherapy regimen alone. No patients experienced dose limiting toxicities, and no discontinuations were observed due to treatment related toxicity. The most common adverse events were white blood cell count decrease and thrombocytopenia (platelet count decrease).
Of the 7 patients enrolled in the Phase 2 lead-in stage of the Harmonic™ trial:
- The clinical benefit rate (CBR) or disease control rate (DCR) was 86% with 6 of the 7 patients receiving clinical benefit to date.
- The preliminary objective response rate (ORR) was 43%, with 3 of the 7 patients having a partial response and 3 patients having stable disease.
- The 3 partial responses include complete disappearance of metastatic lesions and/or normalization of lymph nodes that were abnormal at baseline and the patients experienced an average of 51% reduction in tumor sizes as measured by RECIST criteria.
- In the 3 patients achieving stable disease, there was an average of 13% reduction in tumor size with two patients having distal lesions reduced by 40% in tumor size.
- One patient has been on study for 14 months, showing both a 57% reduction in tumor size and a significant durability of response.
- Data for the remainder (5 of 6) of these patients is not yet mature for estimation of median duration of response and progression free survival (PFS) at the time of data cut off (07/25/24).
- Preliminary efficacy results were observed regardless of prior TKI treatment(s), demographics, or metastatic disease sites, including in patients with identified low or intermediate tumor mutational burden (TMB); a patient group that is particularly unresponsive to existing immuno-oncology therapies.
"These initial results from the Harmonic™ trial provide preliminary clinical evidence of both the safety and the mode of action for LP-300 in never-smokers with NSCLC. To see such a high clinical benefit rate, which is part of our secondary endpoints, this early in the trial is a motivating factor for accelerating our enrollment globally," said Lantern VP of Clinical Development Reggie Ewesuedo, MD, MBA. |
The randomization and expansion phase of the Harmonic™ study is currently ongoing and will assess the Progression Free and Overall Survival (PFS and OS) when patients are treated with pemetrexed and carboplatin (with or without LP-300). The full study design can be viewed here (clinicaltrials.gov).
Other Harmonic Clinical Trial Observations & Updates
Never smokers with NSCLC who progress on treatment with available TKIs are often not candidates for treatment with checkpoint modulator immunotherapy. These patients can often be faced with poor treatment outcomes when receiving the pemetrexed and carboplatin chemotherapy regimen alone, which is the current standard of care for this patient population when failing TKI therapies. This points to a significant clinical need for innovative and new options, especially those that present no overlapping or added toxicities with the standard-of-care chemo doublet.
“Preliminary results indicate that this LP-300 triplet regimen is active against advanced NSCLC with actionable alterations and there were no unexpected adverse events. Also, the early Harmonic patient data indicates that the adverse events appear to be primarily due to chemotherapy and not the study drug,” said Janakiraman Subramanian, MD and Director of Thoracic Oncology at Inova Schar Cancer Institute. |
The proportion of never-smoking patients with non-small cell lung cancer (NSCLC) has been significantly increasing globally over the past 30 years, from 15% in the 1970s to 33% in the 2000s. The high proportion of never smokers with NSCLC in East Asian countries is of particular note with Japan estimated to be 33 to 40% of new cases and Taiwan at over 50% of new cases.3 Lantern has received regulatory approval to initiate the LP-300 clinical trial in multiple Asian countries, and has started activation of sites in Japan and Taiwan, including the National Cancer Center in Tokyo, a globally recognized center of cancer research excellence.
Future Milestones & Activity
Lantern Pharma is committed to further advancing the triplet combination regimen (LP-300 + pemetrexed +carboplatin) to potentially enhance patient outcomes, with the goal of extending and improving the lives of never smokers with advanced NSCLC adenocarcinoma. While this data is promising, Lantern will continue collecting and analyzing additional patient response and clinical data from sites in the US and Asia. If the additional data confirms our preliminary findings for clinical benefit and/or objective response rates, Lantern will consider applying for a Breakthrough Therapy designation. According to the FDA, Breakthrough Therapy designation is a process designed to expedite the development and review of drugs that are intended to treat a serious condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over available therapy on a clinically significant endpoint(s). No therapy has been specifically approved for never-smokers with NSCLC making this a significant and growing global clinical need, estimated by Lantern to be over $2 billion USD in annual market potential.
The Harmonic™ study has progressed to the randomization and expansion phase where up to an additional 80 patients will be placed into either the LP-300 + standard-of-care chemotherapy doublet arm or the standard-of-care chemotherapy doublet arm without LP-300. The arm with LP-300 will enroll patients on a 2 to 1 ratio versus the arm with doublet chemotherapy alone. The study is designed to progress based on the 2 to 1 randomization in all sites across the US and Asia. Lantern plans to review, and share the interim data from, the Phase 2 trial for PFS and OS (co-primary endpoints) after 30 clinical events have been observed.
Lantern also anticipates further using RADR® to determine potential additional suitability for LP-300 in combination with other approved agents for the control of cancer progression in other patient subgroups. Lantern has developed and published on LP-300 as a potential first-in-class combination agent with tyrosine kinase inhibitors (TKIs) in non-smoker lung adenocarcinoma which could extend the potential of the drug-candidate into earlier segments of NSCLC care. On Thursday, August 8th Lantern’s management will provide additional details on the positive Phase 2 trial results as well as clinical and scientific insight from the Harmonic™ study during the Company’s 2nd quarter earnings call at 4:30pm Eastern which can be accessed via Zoom webinar registration.
About The HARMONIC™ Clinical Trial
The HARMONIC™ trial is designed as a multicenter, open label, multi-country Phase 2 trial with planned enrollment of approximately 90 patients. Patients who are never smokers with NSCLC adenocarcinoma and have relapsed after prior treatment with tyrosine kinase inhibitors will be eligible for enrollment. Following the safety lead-in stage, the trial will consist of randomization in a 2:1 allocation ratio of patients to one of two arms: Arm A (LP-300 + carboplatin, + pemetrexed) or Arm B (carboplatin + pemetrexed).
About LP-300
LP-300 is a disulfide small molecule and an investigational new drug candidate. It has been well characterized to have a multimodal mechanism of action directed towards tyrosine kinase receptors and cell redox enzymes. It is believed to modulate cellular redox in key signaling pathways in NSCLC and directly engage with TKI receptors via cysteine modification.
It is known that lung carcinomas in never smoker patients have a much higher percentage of mutations in certain tyrosine kinase (TK) oncogenes such as EGFR, ALK, ROS, and MET-1, contributing to tumor formation and growth, while lung carcinomas in smokers are much more likely to have growth-driver mutations in oncogenes such as RAS, and much lower percentages of mutations in TK oncogenes. Both published (Parker 2015) and unpublished studies have shown that LP-300 covalently binds to and/or inhibits the kinase activity of each of these TK oncogenes (EGFR, ALK, ROS, and MET-1), suggesting that a greater number of lung adenocarcinomas in never smokers, compared to smokers, could be susceptible to the inhibitory effects of LP-300.
LP-300 has been evaluated in 5 Phase 1 and 5 Phase 2 or 3 clinical trials in over 1,000 subjects. In a retrospective subgroup analysis from a prior Phase 3 trial, never smoker lung adenocarcinoma patients receiving the combination of LP-300 with cisplatin and paclitaxel chemotherapy were observed to have significant survival benefit compared to the never smoker patients receiving cisplatin and paclitaxel without LP-300.
About Lantern Pharma:
Lantern Pharma (NASDAQ: LTRN) is an AI company transforming the cost, pace, and timeline of oncology drug discovery and development. Our proprietary AI and machine learning (ML) platform, RADR®, leverages over 100 billion oncology-focused data points and a library of 200+ advanced ML algorithms to help solve billion-dollar, real-world problems in oncology drug development. By harnessing the power of AI and with input from world-class scientific advisors and collaborators, we have accelerated the development of our growing pipeline of therapies that span multiple cancer indications, including both solid tumors and blood cancers and an antibody-drug conjugate (ADC) program. On average, our newly developed drug programs have been advanced from initial AI insights to first-in-human clinical trials in 2-3 years and at approximately $1.0 - 2.5 million per program.
Our lead development programs include a Phase 2 clinical program and multiple Phase 1 clinical trials. We have also established a wholly-owned subsidiary, Starlight Therapeutics, to focus exclusively on the clinical execution of our promising therapies for CNS and brain cancers, many of which have no effective treatment options. Our AI-driven pipeline of innovative product candidates is estimated to have a combined annual market potential of over $15 billion USD and have the potential to provide life-changing therapies to hundreds of thousands of cancer patients across the world.
Please find more information at:
- Website: www.lanternpharma.com
- LinkedIn: https://www.linkedin.com/company/lanternpharma/
- X: @lanternpharma
Forward-looking Statements:
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These forward-looking statements include, among other things, statements relating to: future events or our future financial performance; the potential advantages of our RADR® platform in identifying drug candidates and patient populations that are likely to respond to a drug candidate; our strategic plans to advance the development of our drug candidates and antibody drug conjugate (ADC) development program; estimates regarding the development timing for our drug candidates and ADC development program; expectations and estimates regarding clinical trial timing and patient enrollment; our research and development efforts of our internal drug discovery programs and the utilization of our RADR® platform to streamline the drug development process; our intention to leverage artificial intelligence, machine learning and genomic data to streamline and transform the pace, risk and cost of oncology drug discovery and development and to identify patient populations that would likely respond to a drug candidate; estimates regarding patient populations, potential markets and potential market sizes; sales estimates for our drug candidates and our plans to discover and develop drug candidates and to maximize their commercial potential by advancing such drug candidates ourselves or in collaboration with others. Any statements that are not statements of historical fact (including, without limitation, statements that use words such as "anticipate," "believe," "contemplate," "could," "estimate," "expect," "intend," "seek," "may," "might," "plan," "potential," "predict," "project," "target," “model,” "objective," "aim," "upcoming," "should," "will," "would," or the negative of these words or other similar expressions) should be considered forward-looking statements. There are a number of important factors that could cause our actual results to differ materially from those indicated by the forward-looking statements, such as (i) the risk that our research and the research of our collaborators may not be successful, (ii) the risk that observations in preclinical studies and early or preliminary observations in clinical studies do not ensure that later observations, studies and development will be consistent or successful, (iii) the risk that we may not be successful in licensing potential candidates or in completing potential partnerships and collaborations, (iv) the risk that none of our product candidates has received FDA marketing approval, and we may not be able to successfully initiate, conduct, or conclude clinical testing for or obtain marketing approval for our product candidates, (v) the risk that no drug product based on our proprietary RADR® AI platform has received FDA marketing approval or otherwise been incorporated into a commercial product, and (vi) those other factors set forth in the Risk Factors section in our Annual Report on Form 10-K for the year ended December 31, 2023, filed with the Securities and Exchange Commission on March 18, 2024. You may access our Annual Report on Form 10-K for the year ended December 31, 2023 under the investor SEC filings tab of our website at www.lanternpharma.com or on the SEC's website at www.sec.gov. Given these risks and uncertainties, we can give no assurances that our forward-looking statements will prove to be accurate, or that any other results or events projected or contemplated by our forward-looking statements will in fact occur, and we caution investors not to place undue reliance on these statements. All forward-looking statements in this press release represent our judgment as of the date hereof, and, except as otherwise required by law, we disclaim any obligation to update any forward-looking statements to conform the statement to actual results or changes in our expectations.
| ____________________________________ |
1 The U.S. Centers for Disease Control defines a never-smoker as someone who has smoked < 100 cigarettes per lifetime. A link to the definition is provided here. |
2 Patients in the lead-in cohort of seven patients all received LP-300 in conjunction with carboplatin and pemetrexed intravenously on day one of 21-day cycles for up to four to six cycles. Dosing was established at: LP-300 at 18.4g/m2; pemetrexed at 500mg/m2; and, carboplatin at 5 milligram per milliliter (mg/mL) per minute (AUC5) intravenously on day one of 21-day cycles for four to six cycles. After four to six cycles of therapy (number of cycles was determined by Investigator discretion), patients continue pemetrexed maintenance therapy if patients have evidence of clinical benefit and are not experiencing unacceptable treatment-related toxicity. |
3 Zhou F, Zhou C. Lung cancer in never smokers-the East Asian experience. Transl Lung Cancer Res. 2018 Aug;7(4):450-463. doi: 10.21037/tlcr.2018.05.14. PMID: 30225210; PMCID: PMC6131183. |
View source version on businesswire.com: https://www.businesswire.com/news/home/20240805396240/en/
Contacts
Investor Relations
mailto: ir@lanternpharma.com
ph: (972) 277-1136