Represents the Company’s 24th product launch since inception in October 2023
Unique 5-vial pack supports cost-effective inventory management for healthcare partners
Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, today announced the launch of Dehydrated Alcohol Injection, USP in the United States as a therapeutic generic equivalent of Ablysinol®, which ended its market exclusivity in June 2025.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250721261120/en/
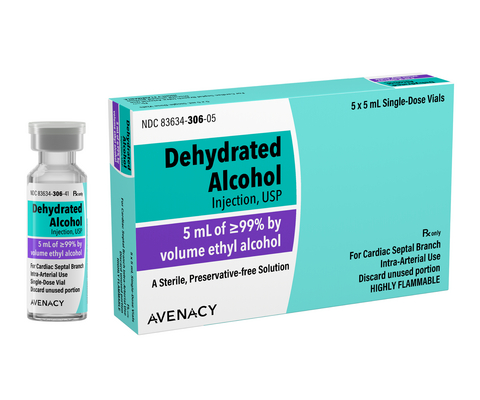
Dehydrated Alcohol Injection is indicated to induce controlled cardiac septal infarction to improve exercise capacity in adults with symptomatic hypertrophic obstructive cardiomyopathy who are not candidates for surgical myectomy.
Each vial of Dehydrated Alcohol Injection contains 5 mL of ethyl alcohol ≥99% by volume and is supplied in a carton of five single-dose vials. Avenacy is currently the only supplier in the U.S. offering this product in a 5-pack configuration, providing healthcare facilities with greater flexibility in controlling inventory expenditures.
“As we continue to add to our rapidly growing portfolio, the launch of Dehydrated Alcohol Injection reflects our strategy of entering high-value markets with practical, differentiated solutions,” said Jeff Yordon, Co-Founder and CEO of Avenacy. “The vial format of Avenacy’s product supports safe handling, and the unique 5-pack presentation helps providers manage high-cost inventory more efficiently, reflecting our company’s longstanding commitment to provide products that meet the needs of both patients and healthcare providers.”
In line with Avenacy’s mission to champion patient safety and streamline patient care, Dehydrated Alcohol Injection will feature the Company’s highly differentiated packaging and labeling to support accurate medication selection.
Avenacy will begin shipping Dehydrated Alcohol Injection, USP to wholesale partners this week. The Company is supported by a global network of development and contract manufacturing partners that have undergone successful FDA inspections based on cGMP standards.
Dehydrated Alcohol Injection, USP had U.S. sales of approximately $71 million for the twelve months ending in March 2025.1
Please see link for Full Prescribing Information.
Ablysinol® is a registered trademark of Belcher Pharmaceuticals, LLC
1 Source: IQVIA
About Avenacy
Avenacy is a U.S.-based specialty pharmaceutical company focused on supplying critical injectable medications used to treat patients in various medically supervised settings, from acute care hospitals to outpatient clinics and physician offices. Through a rigorous and optimized selection process, the company is building a pipeline of high-quality, FDA-approved injectable products to ensure a resilient portfolio that can meet the needs of today’s dynamic drug supply chain. With an experienced team, a commitment to quality and reliability, and product offerings designed to facilitate safe and efficient patient care, Avenacy strives to be a trusted partner for essential medications.
Avenacy was launched in 2023 and is headquartered in Schaumburg, IL. For more information, please visit www.avenacy.com.
View source version on businesswire.com: https://www.businesswire.com/news/home/20250721261120/en/
Contacts
Media Contact
FTI Consulting
Avenacy@fticonsulting.com





