The GLP-1 weight loss trend continues to flourish, and the ante keeps rising. The reigning champions in the battle of the bulge are Novo Nordisk A/V (NYSE: NVO) with its Semaglutide medications Ozempic and Wegovy, and Eli Lilly & Co. (NYSE: LLY) with its Tirzepatide medications Mounjaro and Zepbound. The viral off-label usage for weight loss prompted both companies to rebrand and repurpose their diabetes meds to obesity treatments under new labels with higher doses called Wegovy and Zepbound.
Both medical sector companies are having trouble meeting the insatiable demand to keep their products on the shelves. Additionally, both companies are trying to expand the indications for the use of the drugs beyond diabetes and obesity. Let's review the two obesity treatments, Wegovy and Zepbound, to see how they stack up.
Wegovy
Ozempic made headlines thanks to social media and publicity from celebrities and influencers who swore by its effects on weight loss. Wegovy is a 2.4 mg weekly injection that is FDA-approved specifically for obesity. During its clinical trials, adults taking Wegovy lost an average of 16% of their body weight compared to 2% with the placebo. The 68-week study found that 83% of the participants lost at least 5% of their weight versus 31% of adults taking the placebo. Wegovy is approved for adults and kids 12 and older.
Zepbound
Zepbound is a stronger version of Mounjaro, approved for obesity, but only in adults. The average weight loss was 20.9% after 36 weeks of open-label Zepbound. Zepbound also hit secondary endpoints with improvement in BMI, fast insulin, blood pressure, lipids and health-related quality of life, including sleep apnea. After 88 weeks, Zepbound patients lost an average of 26% of their body weight compared to 9.5% in the placebo group. The percentage of patients who lost at least 20% of their body weight was 72.6% for Zepbound users versus 11.6% for placebo.
However, it's important to point out that in the Surmount-4 study, patients were given 2.5 mg of Zepbound and kept upping the dosage up to their maximum tolerated levels of 10mg or 15mg once weekly. The starting dose was 2.5 mg and was increased by 2.5 mg every 4 weeks until hitting the maximum dosage of 10 mg or 15 mg.
Differences in Mechanisms
While both medications are approved obesity treatments that mimic the GLP-1 hormone, the gut hormone that regulates blood sugar levels and appetite, leading to reduced caloric intake, Zepbound is a dual agonist. It also mimics another cut hormone called glucose-dependent insulinotropic polypeptide (GIP), which works in conjunction with GLP-1 to generate a stronger cumulative effect. This has proven to show greater weight loss.
Differences in Dosing
Just as in the clinical trials, the dosages are also different. Wegovy is suggested to be started at 0.25 mg for 4 weeks. Patients then gradually increase the dosage to 0.5 mg, then 1 mg, then 1.7 mg up to 2.4 mg at the highest dosage.
Zepbound administration starts at 2.5 mg for the first 4 weeks, then increases to 5 mg. Dosage can be increased up to 10 mg or 15 mg based on tolerability.
Differences in Costs
Health insurance coverage varies depending on the carrier. While many insurers cover GLP-1 drugs for type 2 diabetes, they may not cover for obesity. Both medications are once-weekly injections. Wegovy tends to be pricier than Zepbound. GoodRx Health (NASDAQ: GDRX) has a list price for Wegovy at $1,590.29 for a 4-week carton discounted to $1,388.64. It has Zepbound listed at $1,250.08 for a 4-week supply carton discounted to $1,095.66.
Differences in Indications
Wegovy was approved for cardiovascular risk reduction in March 2024. Over 5 years, Wegovy was found to lower the risk of a major adverse cardiovascular event (MACE) by 20%. It was also shown to improve inflammation and walking distances.
Zepbound is effective for fatty liver disease. In a Phase 2 study, 74% of patients taking Zepbound achieved an absence of non-alcoholic steatohepatitis (NASH) with no worsening of the liver fibrosis versus 12.6% for the placebo group. GLP-1 is being studied for stunting the worsening of Parkinson's Disease.
On paper, it appears that Zepbound may be the superior weight loss drug offered at a lower price than Wegovy, acting as a dual agonist mimicking 2 gut hormones instead of just GLP-1. Lilly stock indicates a pullback in the works. However, while Novo Nordisk and Ely Lilly are the kings of the mountains for now, there are many NASDAQ: VKTX">GLP-1 candidates in the works, including VK2735 from Viking Therapeutics Inc. (NASDAQ: VKTX) and Pemvidutide from Altimmune Inc. (NASDAQ: ALT).
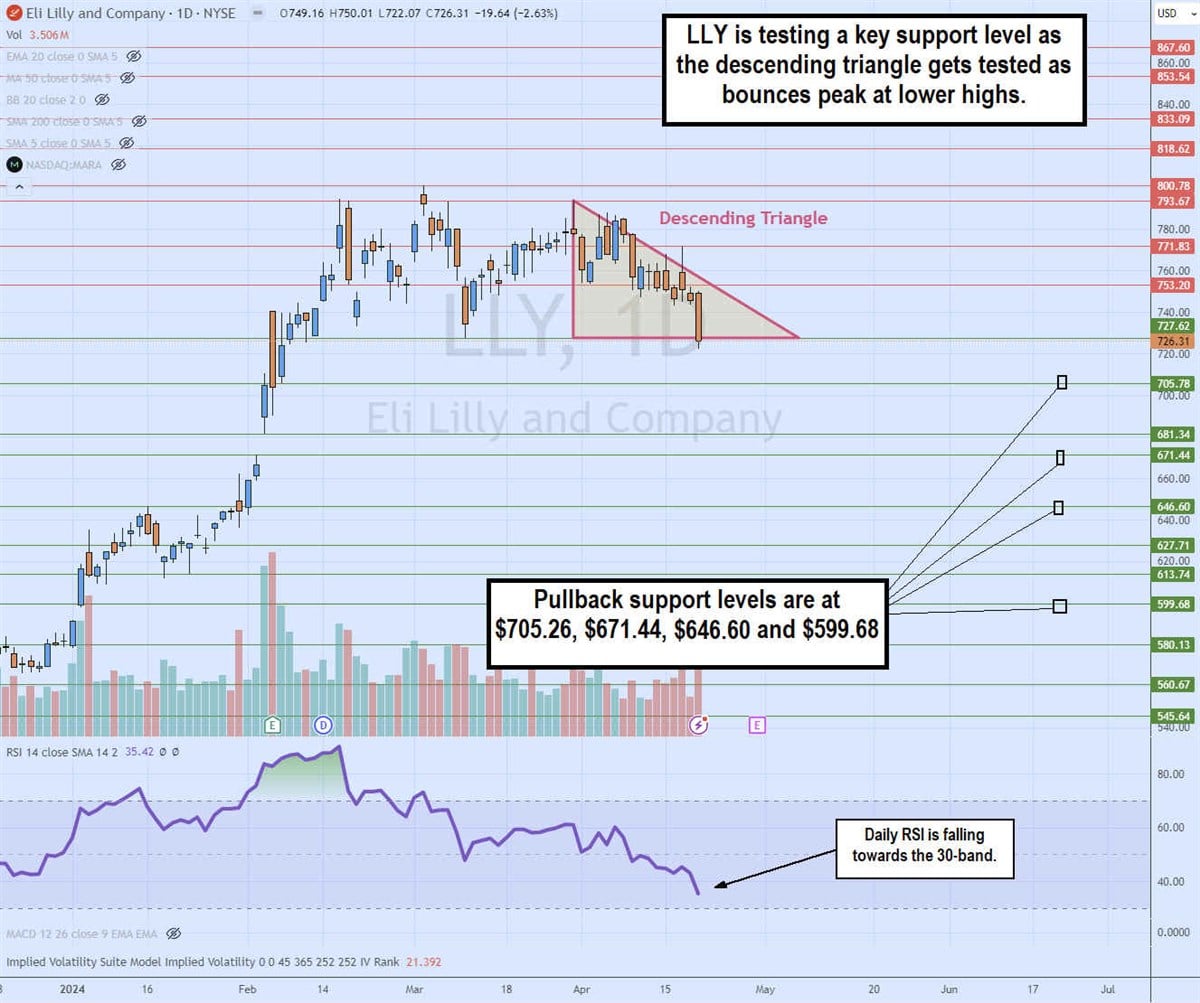
The daily candlestick chart on LLY indicates a descending triangle pattern. The descending trendline formed at $793.67 on March 24, 2025, capping bounces at lower highs. The flat-bottom trendline formed at $726.31. The daily relative strength index (RSI) continues to drift lower toward the 30-band. Pullback support levels are at $705.26, $671.44, $646.60 and $599.68.






