WILMETTE, Ill., Aug. 21, 2024 (GLOBE NEWSWIRE) -- Monopar Therapeutics Inc. (Nasdaq: MNPR), a clinical-stage radiopharma company focused on developing innovative treatments for cancer patients, today announced it has received Human Research Ethics Committee (HREC) clearance in Australia to commence a Phase 1 therapeutic trial of its novel radiopharmaceutical MNPR-101-Lu.
MNPR-101-Lu combines the therapeutic radioisotope lutetium-177 (Lu-177) with Monopar’s proprietary first-in-class humanized monoclonal antibody MNPR-101, which is highly selective against the urokinase plasminogen activator receptor (uPAR). The MNPR-101-Lu Phase 1 clinical trial will enroll patients with advanced solid cancers and will be a therapeutic follow-on study to the currently ongoing MNPR-101-Zr imaging and dosimetry clinical trial.
The results from preclinical studies of MNPR-101-Lu are promising. In a 90-day efficacy study in a human pancreatic cancer xenograft mouse model (Figure 1, below) as an example, MNPR-101-Lu demonstrated durable antitumor effects after a single injection, achieving complete elimination of tumors that lasted the duration of the study.
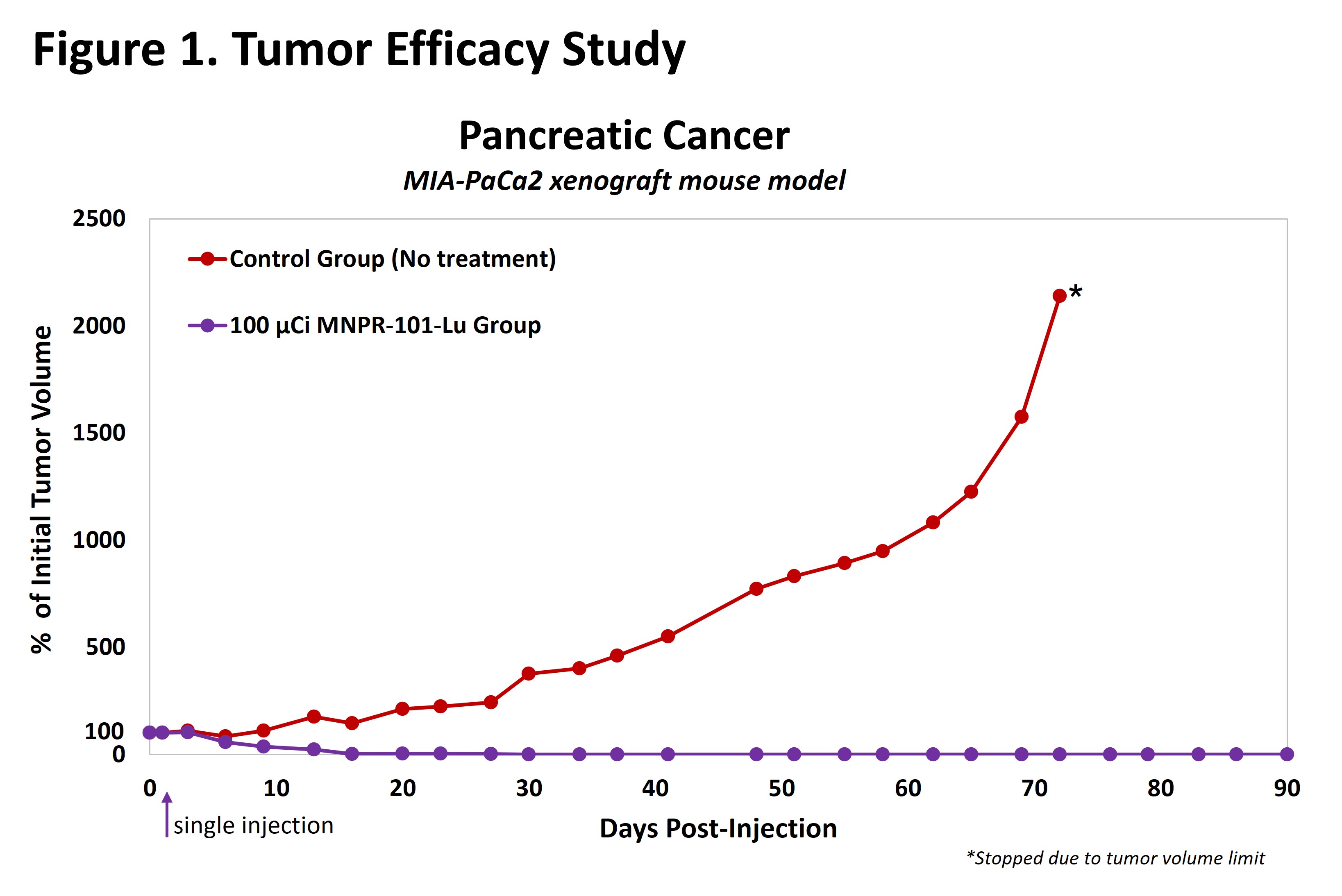
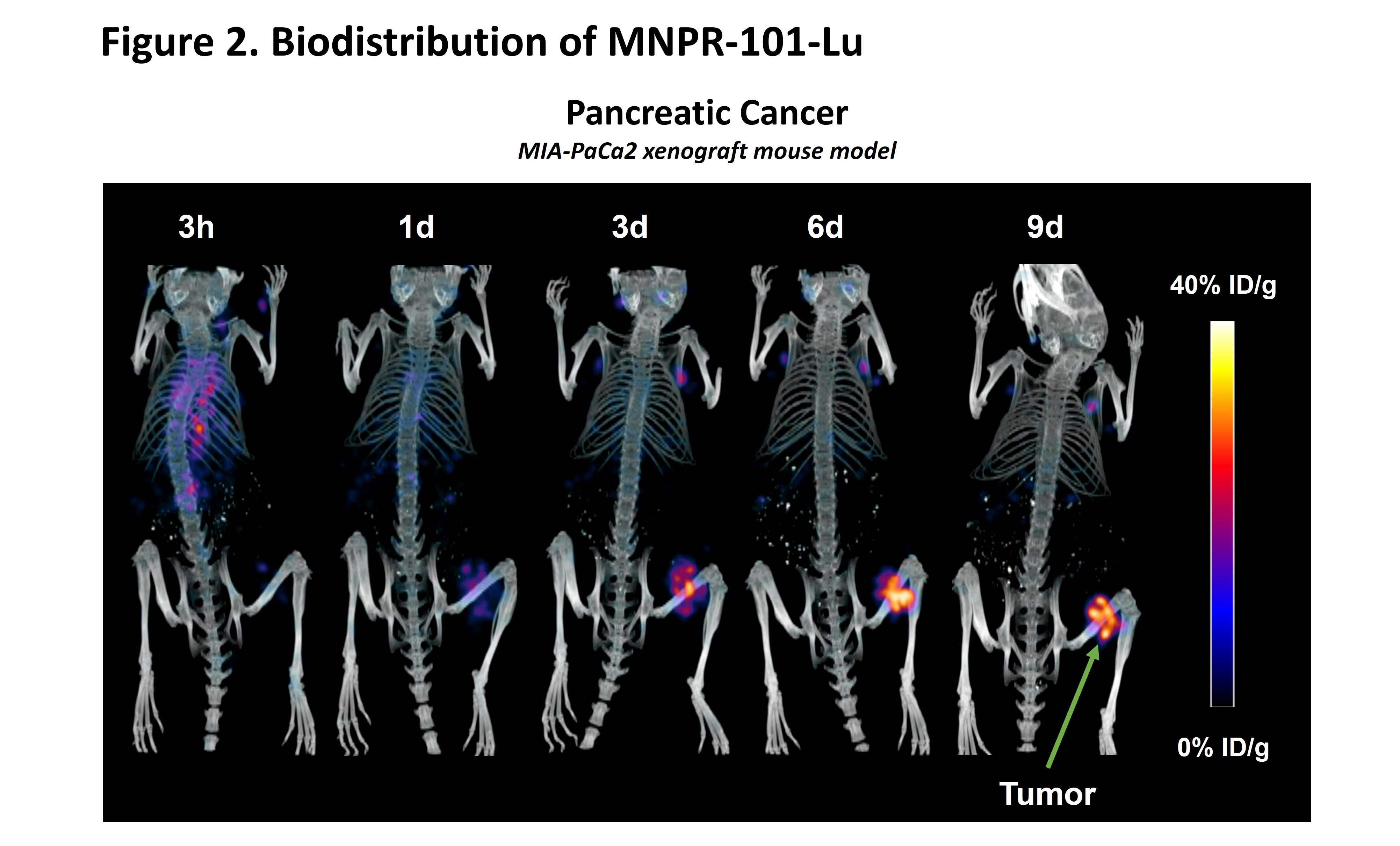
The MNPR-101-Lu imaging data in a human pancreatic cancer xenograft mouse model presented in March (link, Figure 2 below) provides additional insight into the strong therapeutic effect observed after a single injection of MNPR-101-Lu. The imaging data demonstrates the high specificity and durable uptake of MNPR-101-Lu in the tumor relative to normal tissue.
“We are excited about the HREC clearance and encouraged by the potential of MNPR-101-Lu to provide a meaningful clinical benefit to patients with uPAR-positive tumors. Several of the most aggressive, deadly cancers express uPAR, including triple negative breast cancer and pancreatic cancer,” said Chandler Robinson, MD, Monopar’s Chief Executive Officer. “We are looking forward to launching the trial as quickly as we can.”
About Monopar Therapeutics Inc.
Monopar Therapeutics is a clinical-stage radiopharmaceutical company focused on developing innovative treatments for cancer patients, including Phase 1-stage MNPR-101-Zr for imaging advanced cancers and late preclinical-stage MNPR-101-Lu and MNPR-101-Ac225 for the treatment of advanced cancers, as well as early development programs against solid cancers. For more information, visit: www.monopartx.com.
Forward-Looking Statements
Statements contained in this press release regarding matters that are not historical facts are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. The words “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “target” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Examples of these forward-looking statements include: that the MNPR-101-Lu Phase 1 clinical trial will enroll patients with advanced solid cancers, and will be a therapeutic follow-on to the currently ongoing MNPR-101-Zr imaging and dosimetry clinical trial; the results from preclinical studies of MNPR-101-Lu are promising; that these data support the potential of MNPR-101-Lu to provide a meaningful clinical benefit to patients with uPAR-positive tumors; and that Monopar is looking forward to launching the trial as quickly as it can. The forward-looking statements involve risks and uncertainties including, but not limited to: that Monopar may not launch its MNPR-101-Lu therapeutic study even after receiving regulatory clearance; that the Phase 1 imaging and dosimetry clinical trial in advanced cancer patients with MNPR-101-Zr may not yield satisfactory results, if at all; that future preclinical or clinical data will not be as promising as the data to date; that MNPR-101-Zr and/or MNPR-101-Lu may cause unexpected serious adverse effects or fail to image or be effective against the cancer tumors in humans; and the significant general risks and uncertainties surrounding the research, development, regulatory approval, and commercialization of imaging agents and therapeutics. Actual results may differ materially from those expressed or implied by such forward-looking statements. Risks are described more fully in Monopar's filings with the Securities and Exchange Commission. All forward-looking statements contained in this press release speak only as of the date on which they were made. Monopar undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made. Any forward-looking statements contained in this press release represent Monopar’s views only as of the date hereof and should not be relied upon as representing its views as of any subsequent date.
CONTACT:
Monopar Therapeutics Inc.
Investor Relations
Karthik Radhakrishnan
Chief Financial Officer
karthik@monopartx.com
Follow Monopar on social media for updates:
Twitter: @MonoparTx LinkedIn: Monopar Therapeutics
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/46065fd5-9a23-4688-9bbe-1bdb6e74954e
https://www.globenewswire.com/NewsRoom/AttachmentNg/6fe00a55-f6db-4fba-a0b3-6d4f474b517c








